Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: A prespecified subgroup analysis of the randomized, placebo-controlled CARMELINA® trial
- PMID: 32037653
- PMCID: PMC7317902
- DOI: 10.1111/dom.13995
Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: A prespecified subgroup analysis of the randomized, placebo-controlled CARMELINA® trial
Abstract
Aims: In CARMELINA®, linagliptin demonstrated cardiovascular and renal safety in patients with type 2 diabetes (T2D) with high renal and cardiovascular disease (CVD) risk. We investigated safety and efficacy of this dipeptidyl peptidase-4 inhibitor in older participants.
Materials and methods: Subjects aged ≥18 years with T2D and established CVD with urinary albumin-to-creatinine ratio (UACR) >30 mg/g, and/or prevalent kidney disease, were randomized to linagliptin or placebo added to usual care. The primary endpoint (time to first occurrence of 3P-MACE: cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) and other outcomes were evaluated across age groups <65 (n = 2968), 65 to <75 (n = 2800) and ≥75 years (n = 1211).
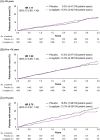
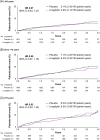
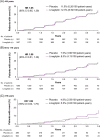
Results: Mean age was 65.9 years (17.4% and 5.9% aged ≥75 and 80, respectively) and median follow-up was 2.2 years. The hazard ratio (HR) for 3P-MACE with linagliptin versus placebo was 1.02 [95% confidence interval (CI) 0.89, 1.17] with no significant interaction between age and treatment effect (P = 0.0937). HRs for participants aged <65, 65 to <75 and ≥75 years were 1.11 (95% CI 0.89, 1.40), 1.09 (0.89, 1.33) and 0.76 (0.57, 1.02), respectively. Linagliptin did not increase the risk of adverse kidney outcomes or hospitalization for heart failure across age groups. The incidence of adverse events, including hypoglycaemia, increased with age but was similar with linagliptin and placebo despite glycated haemoglobin A1c reduction with linagliptin.
Conclusions: Linagliptin did not increase risk for cardiovascular events or hypoglycaemia and kidney function remained stable in older people with T2D and established CVD with albuminuria and/or kidney disease.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
M.E.C. has received fees for advisory services and honoraria from Boehringer Ingelheim, Eli Lilly, Sanofi, Servier, Bayer, Astra Zeneca, Novartis, Reata, MundiPharma and MSD, and a grant from NovoNordisk. J.R. has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, Applied Therapeutics, Boehringer Ingelheim, and Intarcia; he has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon, Boehringer Ingelheim, Oramed, Applied Therapeutics and Intarcia. T.K. reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd, Kissei Pharmaceutical Co., Ltd, Kowa Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., Sanwa Kagaku Kenkyusho Co., Ltd, Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd, Takeda Pharmaceutical Company Limited, and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited; contracted research from AstraZeneca K.K. and Takeda Pharmaceutical Company Limited; joint research from Daiichi Sankyo Company, Limited; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited. Y.S. has received lecture fees from MSD, K.K., Kao, Taisho, Boehringer Ingelheim, Taisho Toyama, Takeda, Becton Dickinson and Novo Nordisk; and research support from Terumo, Bayer, Boehringer Ingelheim, Ono, Sumitomo Dainippon, Taisho Toyama and Novo Nordisk. C.W. has received fees for advisory services to Boehringer Ingelheim and MSD as well as honoraria for lecturing from AstraZeneca, Eli Lilly and Sanofi. S.S., D.C. and O.E.J. are employees of Boehringer Ingelheim.
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017.
-
- Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011.
-
- Lakey WC, Barnard K, Batch BC, Chiswell K, Tasneem A, Green JB. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia. 2013;56:1226‐1235. - PubMed
-
- Tong L, Adler S. Glycemic control of type 2 diabetes mellitus across stages of renal impairment: information for primary care providers. Postgrad Med. 2018;130:381‐393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

