The Many Facets of Erythropoietin Physiologic and Metabolic Response
- PMID: 32038269
- PMCID: PMC6984352
- DOI: 10.3389/fphys.2019.01534
The Many Facets of Erythropoietin Physiologic and Metabolic Response
Abstract
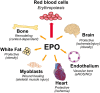
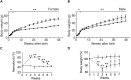
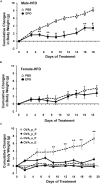
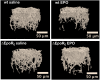
In mammals, erythropoietin (EPO), produced in the kidney, is essential for bone marrow erythropoiesis, and hypoxia induction of EPO production provides for the important erythropoietic response to ischemic stress, such as during blood loss and at high altitude. Erythropoietin acts by binding to its cell surface receptor which is expressed at the highest level on erythroid progenitor cells to promote cell survival, proliferation, and differentiation in production of mature red blood cells. In addition to bone marrow erythropoiesis, EPO causes multi-tissue responses associated with erythropoietin receptor (EPOR) expression in non-erythroid cells such neural cells, endothelial cells, and skeletal muscle myoblasts. Animal and cell models of ischemic stress have been useful in elucidating the potential benefit of EPO affecting maintenance and repair of several non-hematopoietic organs including brain, heart and skeletal muscle. Metabolic and glucose homeostasis are affected by endogenous EPO and erythropoietin administration affect, in part via EPOR expression in white adipose tissue. In diet-induced obese mice, EPO is protective for white adipose tissue inflammation and gives rise to a gender specific response in weight control associated with white fat mass accumulation. Erythropoietin regulation of fat mass is masked in female mice due to estrogen production. EPOR is also expressed in bone marrow stromal cells (BMSC) and EPO administration in mice results in reduced bone independent of the increase in hematocrit. Concomitant reduction in bone marrow adipocytes and bone morphogenic protein suggests that high EPO inhibits adipogenesis and osteogenesis. These multi-tissue responses underscore the pleiotropic potential of the EPO response and may contribute to various physiological manifestations accompanying anemia or ischemic response and pharmacological uses of EPO.
Keywords: bone; erythropoietin; erythropoietin receptor; gender-specific; inflammation; nitric oxide; obesity.
Copyright © 2020 Suresh, Rajvanshi and Noguchi.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

