Vascular Integrity and Signaling Determining Brain Development, Network Excitability, and Epileptogenesis
- PMID: 32038280
- PMCID: PMC6987412
- DOI: 10.3389/fphys.2019.01583
Vascular Integrity and Signaling Determining Brain Development, Network Excitability, and Epileptogenesis
Abstract
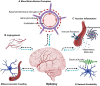
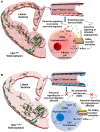
Our understanding of the etiological mechanisms leading up to epilepsy has undergone radical changes over time due to more insights into the complexity of the disease. The traditional hypothesis emphasized network hyperexcitability and an imbalance of inhibition and excitation, eventually leading to seizures. In this context, the contribution of the vascular system, and particularly the interactions between blood vessels and neuronal tissue, came into focus only recently. Thus, one highly exciting causative or contributing factor of epileptogenesis is the disruption of the blood-brain barrier (BBB) in the context of not only posttraumatic epilepsy, but also other etiologies. This hypothesis is now recognized as a synergistic mechanism that can give rise to epilepsy, and BBB repair for restoration of cerebrovascular integrity is considered a therapeutic alternative. Endothelial cells lining the inner surface of blood vessels are an integral component of the BBB system. Sealed by tight junctions, they are crucial in maintaining homeostatic activities of the brain, as well as acting as an interface in the neurovascular unit. Additional potential vascular mechanisms such as inflammation, altered neurovascular coupling, or changes in blood flow that can modulate neuronal circuit activity have been implicated in epilepsy. Our own work has shown how intrinsic defects within endothelial cells from the earliest developmental time points, which preclude neuronal changes, can lead to vascular abnormalities and autonomously support the development of hyperexcitability and epileptiform activity. In this article, we review the importance of vascular integrity and signaling for network excitability and epilepsy by highlighting complementary basic and clinical research studies and by outlining possible novel therapeutic strategies.
Keywords: blood-brain barrier; development; epileptogenesis; hyperexcitability; inflammation; vascular endothelia.
Copyright © 2020 Baruah, Vasudevan and Köhling.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

