Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio)
- PMID: 32038507
- PMCID: PMC6988821
- DOI: 10.3389/fmicb.2019.02742
Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio)
Abstract
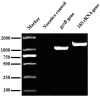
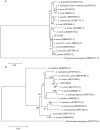
Aeromonas species often cause disease in farmed fish. In the present study, dominant bacteria were isolated from diseased crucian carp (Carassius auratus gibelio). Based on this, a bacterial isolate was tentatively named CFJY-623. This isolate was identified as Aeromonas veronii based on analysis of its morphological, physiological, and biochemical features, as well as 16S rRNA and gyrB gene sequences. Six virulence genes related to pathogenicity including aerolysin, cytotonic enterotoxins, elastase, glycerophospholipid: cholesterol acyltransferase, lipase, and serine protease were identified in this A. veronii isolate. The median lethal dosage (LD50) of the CFJY-623 isolate for crucian carp was determined as 1.31 × 107 CFU/mL. Artificial experimental infection showed that the CFJY-623 isolate caused considerable histological lesions in the fish, including tissue cell degeneration, necrosis, and inflammatory cell infiltrating. Drug sensitivity testing showed that the isolate was susceptible to aminoglycosides, carbapenemes, and nitrofurans. Exploring its growing features showed that this isolate exhibited a high level of environmental adaptability. These results provided a scientific basis for the identification of A. veronii and treatment for fish infected by this pathogen.
Keywords: 16S rRNA gene; Aeromonas veronii; Carassius auratus gibelio; growing characteristics; gyrB gene; virulence genes.
Copyright © 2019 Chen, Sun, Han, Yang, Xian, Lv, Hu and Shi.
Figures




References
-
- Aguilera-Arreola M. G., Hernández-Rodríguez C., Zúñiga G., Figueras M. J., Garduño R. A., Castro-Escarpulli G. (2007). Virulence potential and genetic diversity of Aeromonas caviae, Aeromonas veronii, and Aeromonas hydrophila clinical isolates from Mexico and Spain: a comparative study. Can. J. Microbiol. 53 877–887. 10.1139/W07-051 - DOI - PubMed
-
- Chandrarathna H. P. S. U., Nikapitiya C., Dananjaya S. H. S., Wijerathne C. U. B., Wimalasena S. H. M. P., Kwun H. J., et al. (2018). Outcome of co-infection with opportunistic and multidrug resistant, Aeromonas hydrophila and A. veronii, in zebrafish: identification, characterization, pathogenicity and immune responses. Fish Shellfish Immunol. 80 573–581. 10.1016/j.fsi.2018.06.049 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

