Degradation of Recalcitrant Polyurethane and Xenobiotic Additives by a Selected Landfill Microbial Community and Its Biodegradative Potential Revealed by Proximity Ligation-Based Metagenomic Analysis
- PMID: 32038514
- PMCID: PMC6987047
- DOI: 10.3389/fmicb.2019.02986
Degradation of Recalcitrant Polyurethane and Xenobiotic Additives by a Selected Landfill Microbial Community and Its Biodegradative Potential Revealed by Proximity Ligation-Based Metagenomic Analysis
Abstract
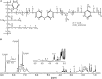
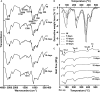
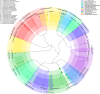
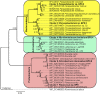
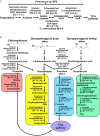
Polyurethanes (PU) are the sixth most produced plastics with around 18-million tons in 2016, but since they are not recyclable, they are burned or landfilled, generating damage to human health and ecosystems. To elucidate the mechanisms that landfill microbial communities perform to attack recalcitrant PU plastics, we studied the degradative activity of a mixed microbial culture, selected from a municipal landfill by its capability to grow in a water PU dispersion (WPUD) as the only carbon source, as a model for the BP8 landfill microbial community. The WPUD contains a polyether-polyurethane-acrylate (PE-PU-A) copolymer and xenobiotic additives (N-methylpyrrolidone, isopropanol and glycol ethers). To identify the changes that the BP8 microbial community culture generates to the WPUD additives and copolymer, we performed chemical and physical analyses of the biodegradation process during 25 days of cultivation. These analyses included Nuclear magnetic resonance, Fourier transform infrared spectroscopy, Thermogravimetry, Differential scanning calorimetry, Gel permeation chromatography, and Gas chromatography coupled to mass spectrometry techniques. Moreover, for revealing the BP8 community structure and its genetically encoded potential biodegradative capability we also performed a proximity ligation-based metagenomic analysis. The additives present in the WPUD were consumed early whereas the copolymer was cleaved throughout the 25-days of incubation. The analysis of the biodegradation process and the identified biodegradation products showed that BP8 cleaves esters, C-C, and the recalcitrant aromatic urethanes and ether groups by hydrolytic and oxidative mechanisms, both in the soft and the hard segments of the copolymer. The proximity ligation-based metagenomic analysis allowed the reconstruction of five genomes, three of them from novel species. In the metagenome, genes encoding known enzymes, and putative enzymes and metabolic pathways accounting for the biodegradative activity of the BP8 community over the additives and PE-PU-A copolymer were identified. This is the first study revealing the genetically encoded potential biodegradative capability of a microbial community selected from a landfill, that thrives within a WPUD system and shows potential for bioremediation of polyurethane- and xenobiotic additives-contamitated sites.
Keywords: Hi-C proximity-ligation; biodegradation; biodegradative potential; community structure; metagenomics; microbial community; polyether-polyurethane-acrylate; xenobiotic additives.
Copyright © 2020 Gaytán, Sánchez-Reyes, Burelo, Vargas-Suárez, Liachko, Press, Sullivan, Cruz-Gómez and Loza-Tavera.
Figures


References
-
- Albertsson A. C., Erlandsson B., Hakkarainen M., Karlsson S. (1998). Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegraded polyethylene. J. Environ. Pol. Degrad. 6 187–195. 10.1023/A:1021873631162 - DOI
-
- Álvarez-Barragán J., González-Hernández R., Domínguez-Malfavón L., Vargas-Suárez M., Aguilar-Osorio G., Loza-Tavera H. (2016). Biodegradative activity of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl. Environ. Microbiol. 82 5225–5235. 10.1128/AEM.01344-1316 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

