Shedding Light on the Cell Biology of the Predatory Bacterium Bdellovibrio bacteriovorus
- PMID: 32038570
- PMCID: PMC6985089
- DOI: 10.3389/fmicb.2019.03136
Shedding Light on the Cell Biology of the Predatory Bacterium Bdellovibrio bacteriovorus
Abstract
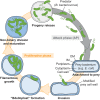
Bdellovibrio bacteriovorus is a predatory bacterium that feeds upon and proliferates inside other Gram-negative bacteria. Upon entry into the periplasmic space of the prey envelope, B. bacteriovorus initiates an exquisite developmental program in which it digests the host resources and grows as a filament, which eventually divides in a non-binary manner, releasing a variable number of daughter cells. The progeny then escape from the prey ghost to encounter new victims and resume the predation cycle. Owing to its unique biology, B. bacteriovorus undoubtedly represents an attractive model to unravel novel mechanisms of bacterial cell cycle control and cellular organization. Yet, the molecular factors behind the sophisticated lifestyle of this micro-predator are still mysterious. In particular, the spatiotemporal dynamics of proteins that control key cellular processes such as transmission of the genetic information, cell growth and division remain largely unexplored. In this Perspective article, I highlight outstanding fundamental questions related to these aspects and arising from the original biology of this bacterium. I also discuss available insights and potential cell biology approaches based on quantitative live imaging techniques, in combination with bacterial genetics and biochemistry, to shed light on the intracellular organization of B. bacteriovorus in space and time.
Keywords: Bdellovibrio bacteriovorus; bacterial cell biology; cell cycle; cell division; chromosome dynamics; microscopy; polarity; predation.
Copyright © 2020 Laloux.
Figures
References
LinkOut - more resources
Full Text Sources

