Detection of EXP1-Specific CD4+ T Cell Responses Directed Against a Broad Range of Epitopes Including Two Promiscuous MHC Class II Binders During Acute Plasmodium falciparum Malaria
- PMID: 32038611
- PMCID: PMC6993587
- DOI: 10.3389/fimmu.2019.03037
Detection of EXP1-Specific CD4+ T Cell Responses Directed Against a Broad Range of Epitopes Including Two Promiscuous MHC Class II Binders During Acute Plasmodium falciparum Malaria
Abstract
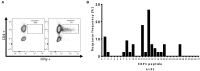
Background: T cells are thought to play a major role in conferring immunity against malaria. This study aimed to comprehensively define the breadth and specificity of the Plasmodium falciparum (P. falciparum)-specific CD4+ T cell response directed against the exported protein 1 (EXP1) in a cohort of patients diagnosed with acute malaria. Methods: Peripheral blood mononuclear cells of 44 patients acutely infected with P. falciparum, and of one patient infected with P. vivax, were stimulated and cultured in vitro with an overlapping set of 31 P. falciparum-specific 13-17-mer peptides covering the entire EXP1 sequence. EXP1-specific T cell responses were analyzed by ELISPOT and intracellular cytokine staining for interferon-γ production after re-stimulation with individual peptides. For further characterization of the epitopes, in silico and in vitro human leukocyte antigen (HLA) binding studies and fine mapping assays were performed. Results: We detected one or more EXP1-specific CD4+ T cell responses (mean: 1.09, range 0-5) in 47% (21/45) of our patients. Responses were directed against 15 of the 31 EXP1 peptides. Peptides EXP1-P13 (aa60-74) and P15 (aa70-85) were detected by 18% (n = 8) and 27% (n = 12) of the 45 patients screened. The optimal length, as well as the corresponding most likely HLA-restriction, of each of these two peptides was assessed. Interestingly, we also identified one CD4+ T cell response against peptide EXP1-P15 in a patient who was infected with P. vivax but not falciparum. Conclusions: This first detailed characterization of novel EXP1-specific T cell epitopes provides important information for future analysis with major histocompatibility complex-multimer technology as well as for immunomonitoring and vaccine design.
Keywords: CD4+; CD8+; HLA binding; HLA class II; Plasmodium falciparum; Plasmodium vivax; T cell epitope; malaria.
Copyright © 2020 Heide, Wildner, Ackermann, Wittner, Marget, Sette, Sidney, Jacobs and Schulze zur Wiesch.
Figures


Similar articles
-
Bridging Computational Vaccinology and Vaccine Development Through Systematic Identification, Characterization, and Downselection of Conserved and Variable Circumsporozoite Protein CD4 T Cell Epitopes From Diverse Plasmodium falciparum Strains.Front Immunol. 2021 Jun 8;12:689920. doi: 10.3389/fimmu.2021.689920. eCollection 2021. Front Immunol. 2021. PMID: 34168657 Free PMC article.
-
Identification and Immune Assessment of T Cell Epitopes in Five Plasmodium falciparum Blood Stage Antigens to Facilitate Vaccine Candidate Selection and Optimization.Front Immunol. 2021 Jul 7;12:690348. doi: 10.3389/fimmu.2021.690348. eCollection 2021. Front Immunol. 2021. PMID: 34305923 Free PMC article.
-
Identification of minimal CD8+ and CD4+ T cell epitopes in the Plasmodium yoelii hepatocyte erythrocyte protein 17kDa.Mol Immunol. 2007 Apr;44(11):3037-48. doi: 10.1016/j.molimm.2007.01.001. Epub 2007 Feb 15. Mol Immunol. 2007. PMID: 17303242
-
Comprehensive Review of Human Plasmodium falciparum-Specific CD8+ T Cell Epitopes.Front Immunol. 2019 Mar 21;10:397. doi: 10.3389/fimmu.2019.00397. eCollection 2019. Front Immunol. 2019. PMID: 30949162 Free PMC article. Review.
-
Using two phases of the CD4 T cell response to blood-stage murine malaria to understand regulation of systemic immunity and placental pathology in Plasmodium falciparum infection.Immunol Rev. 2020 Jan;293(1):88-114. doi: 10.1111/imr.12835. Immunol Rev. 2020. PMID: 31903675 Free PMC article. Review.
Cited by
-
Broadly directed SARS-CoV-2-specific CD4+ T cell response includes frequently detected peptide specificities within the membrane and nucleoprotein in patients with acute and resolved COVID-19.PLoS Pathog. 2021 Sep 16;17(9):e1009842. doi: 10.1371/journal.ppat.1009842. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34529740 Free PMC article.
-
Adaptive Immune Responses, Immune Escape and Immune-Mediated Pathogenesis during HDV Infection.Viruses. 2022 Jan 20;14(2):198. doi: 10.3390/v14020198. Viruses. 2022. PMID: 35215790 Free PMC article. Review.
-
Epitope mapping of Acinetobacter baumannii outer membrane protein W (OmpW) and laboratory study of an OmpW-derivative peptide.Heliyon. 2023 Jul 25;9(8):e18614. doi: 10.1016/j.heliyon.2023.e18614. eCollection 2023 Aug. Heliyon. 2023. PMID: 37560650 Free PMC article.
-
Deciphering the Plasmodium falciparum malaria-specific CD4+ T-cell response: ex vivo detection of high frequencies of PD-1+TIGIT+ EXP1-specific CD4+ T cells using a novel HLA-DR11-restricted MHC class II tetramer.Clin Exp Immunol. 2022 Apr 4;207(2):227-236. doi: 10.1093/cei/uxab027. Clin Exp Immunol. 2022. PMID: 35020841 Free PMC article.
-
High-resolution analysis of individual spike peptide-specific CD4+ T-cell responses in vaccine recipients and COVID-19 patients.Clin Transl Immunology. 2022 Aug 9;11(8):e1410. doi: 10.1002/cti2.1410. eCollection 2022. Clin Transl Immunology. 2022. PMID: 35957961 Free PMC article.
References
-
- World Health Organization World Malaria Report 2018. (2018). Available at: www.who.int/malaria
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

