MicroRNA-1 Negatively Regulates Peripheral NK Cell Function via Tumor Necrosis Factor-Like Weak Inducer of Apoptosis (TWEAK) Signaling Pathways During PPRV Infection
- PMID: 32038620
- PMCID: PMC6989477
- DOI: 10.3389/fimmu.2019.03066
MicroRNA-1 Negatively Regulates Peripheral NK Cell Function via Tumor Necrosis Factor-Like Weak Inducer of Apoptosis (TWEAK) Signaling Pathways During PPRV Infection
Abstract
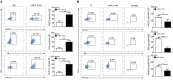
Peste des petits ruminants virus (PPRV) has emerged as a significant threat to the productivity of small ruminants worldwide. PPRV is lymphotropic in nature and induces in the hosts a transient but severe immunosuppression, especially innate immunity. However, it remains largely unknown how NK cells respond and are regulated at the earliest time points after an acute viral PPRV infection in goats. In this study, we revealed that multiple immune responses of goat peripheral NK cells were compromised during PPRV infection, including the cytolytic effector molecule expression and cytokine production. Importantly, we demonstrated that PPRV infection stimulated the expression of TWEAK, a negative regulator of cytotoxic function of NK cells, which may be involved in the suppression of cytotoxicity as well as cytokine production in infected goat NK cells. Furthermore, we found that PPRV infection induced TWEAK expression in goat NK cells involving post-transcription by suppressing miR-1, a novel negative miRNA directly targeting the TWEAK gene. Moreover, replication of virus is required for inhibition of miR-1 expression during PPRV infection, and the non-structural V protein of PPRV plays an important role in miR-1 mediated TWEAK upregulation. Additionally, we revealed that the regulation of NK cell immune responses by TWEAK is mediated by MyD88, SOCS1, and STAT3. Taken together, our results demonstrated that TWEAK may play a key role in regulating goat peripheral NK cell cytotoxicity and cytokine expression levels during PPRV infection.
Keywords: MicroRNA-1; NK cells; PPRV; TWEAK; goat.
Copyright © 2020 Qi, Li, Li, Wang, Zhang and Wang.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

