The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity
- PMID: 32038642
- PMCID: PMC6987434
- DOI: 10.3389/fimmu.2019.03133
The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity
Abstract
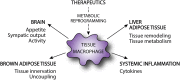
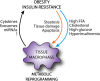
Obesity is associated with the development of metabolic diseases such as type 2 diabetes and non-alcoholic fatty liver disease. The presence of chronic, low-grade inflammation appears to be an important mechanistic link between excess nutrients and clinical disease. The onset of these metabolic disorders coincides with changes in the number and phenotype of macrophages in peripheral organs, particularly in the liver and adipose tissue. Macrophage accumulation in these tissues has been implicated in tissue inflammation and fibrosis, contributing to metabolic disease progression. Recently, the concept has emerged that changes in macrophage metabolism affects their functional phenotype, possibly triggered by distinct environmental metabolic cues. This may be of particular importance in the setting of obesity, where both liver and adipose tissue are faced with a high metabolic burden. In the first part of this review we will discuss current knowledge regarding macrophage dynamics in both adipose tissue and liver in obesity. Then in the second part, we will highlight data linking macrophage metabolism to functional phenotype with an emphasis on macrophage activation in metabolic disease. The importance of understanding how tissue niche influences macrophage function in obesity will be highlighted. In addition, we will identify important knowledge gaps and outstanding questions that are relevant for future research in this area and will facilitate the identification of novel targets for therapeutic intervention in associated metabolic diseases.
Keywords: adipose tissue; insulin resistance; liver; macrophages; metabolism; non-alcoholic fatty liver disease; obesity.
Copyright © 2020 Daemen and Schilling.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

