Cancer Cells Expressing Oncogenic Rat Sarcoma Show Drug-Addiction Toward Epidermal Growth Factor Receptor Antibodies Mediated by Sustained MAPK Signaling
- PMID: 32039027
- PMCID: PMC6985072
- DOI: 10.3389/fonc.2019.01559
Cancer Cells Expressing Oncogenic Rat Sarcoma Show Drug-Addiction Toward Epidermal Growth Factor Receptor Antibodies Mediated by Sustained MAPK Signaling
Abstract
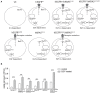
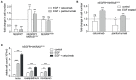
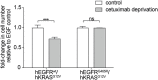
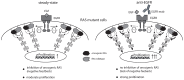
Epidermal growth factor receptor (EGFR) antibodies may have detrimental effects in patients with metastatic colorectal cancer expressing oncogenic Rat sarcoma (RAS). Since a significant number of patients acquire RAS-mediated resistance during EGFR-directed treatment, understanding the molecular mechanism underlying these antibody-mediated tumor-promoting effects is of relevance to design more resistance-preventive treatment approaches. To test this, we set up a Ba/F3 cellular model system transformed to EGFR/RAS dependency to be able to study proliferation, RAS activity as well as MAPK signaling upon inhibition of wild-type RAS isoforms by therapeutic EGFR antibodies. Here, we show that the EGFR antibodies cetuximab and panitumumab induce paradoxical stimulation and enhance proliferation in cells expressing oncogenic RAS (KRAS G12V). These experiments clearly showed that the stimulatory effect is a direct result of the antibody-EGFR interaction leading to prolonged mitogen-activated protein-Kinase (MAPK) signaling. The effect was also induced by antibody-chemotherapy combinations but always depended on simultaneous low-level ligand-dependent EGFR pathway activation. Moreover, we observed significant growth retardation of RAS mutant cells after antibody withdrawal compatible with a drug-addiction phenotype. Our data suggests that EGFR antibodies paradoxically sustain MAPK signaling downstream of oncogenic RAS thereby driving proliferation of RAS mutant tumors or tumor subclones. The observed drug-addiction encourages fixed-duration or liquid-biopsy-guided drug holiday concepts to preventively clear RAS mutant subclones selected under EGFR-directed therapeutic pressure.
Keywords: EGFR antibodies; MAPK signaling; acquired resistance; colorectal cancer; oncogenic RAS.
Copyright © 2020 Tintelnot, Metz, Trentmann, Oberle, von Wenserski, Schultheiß, Braig, Kriegs, Fehse, Riecken, Bokemeyer, Stein and Binder.
Figures


References
-
- Gill GN, Kawamotoli T, Cochets C, Le A, Sato JD, Masui H, et al. . Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. (1984) 259:7755–60. - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. . Randomized, Phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) Versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. (2010) 28:4697–705. 10.1200/JCO.2009.27.4860 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

