From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options
- PMID: 32039382
- PMCID: PMC7001557
- DOI: 10.1016/j.jhepr.2019.07.002
From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options
Abstract
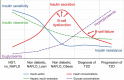
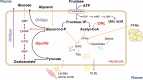
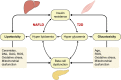
The worldwide prevalence of non-alcoholic fatty liver disease (NAFLD) is estimated to have reached 25% or more in adults. NAFLD is prevalent in obese individuals, but may also affect non-obese insulin-resistant individuals. NAFLD is associated with a 2- to 3-fold increased risk of developing type 2 diabetes (T2D), which may be higher in patients with more severe liver disease - fibrosis increases this risk. In NAFLD, not only the close association with obesity, but also the impairment of many metabolic pathways, including decreased hepatic insulin sensitivity and insulin secretion, increase the risk of developing T2D and related comorbidities. Conversely, patients with diabetes have a higher prevalence of steatohepatitis, liver fibrosis and end-stage liver disease. Genetics and mechanisms involving dysfunctional adipose tissue, lipotoxicity and glucotoxicity appear to play a role. In this review, we discuss the altered pathophysiological mechanisms that underlie the development of T2D in NAFLD and vice versa. Although there is no approved therapy for the treatment of NASH, we discuss pharmacological agents currently available to treat T2D that could potentially be useful for the management of NASH.
© 2019 The Authors.
Figures


References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- EASL, Marchesini G, Day CP, Dufour J-F, Canbay A, Nobili V. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. - PubMed
-
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. - PubMed
-
- Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372–382. - PubMed
-
- Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

