Management of patients with pre-therapeutic advanced liver fibrosis following HCV eradication
- PMID: 32039400
- PMCID: PMC7005771
- DOI: 10.1016/j.jhepr.2019.11.001
Management of patients with pre-therapeutic advanced liver fibrosis following HCV eradication
Abstract
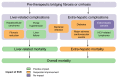
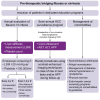
Patients with HCV-related bridging fibrosis or cirrhosis remain at risk of developing life-threatening complications even after achieving a sustained virological response. Although it is reduced, the risk of liver-related events in these patients justifies their inclusion in surveillance programmes dedicated to the early detection of hepatocellular carcinoma and the screening for portal hypertension. Biochemical parameters or non-invasive tests might indicate the potential progression of liver injury despite viral clearance. Specific attention must be focused on the management of comorbidities, while dedicated educational programmes must be encouraged to increase compliance and commitment to surveillance. Better knowledge of the long-term evolution of these patients, who now live longer, is essential to improve risk stratification and refine screening strategies in this growing population.
Keywords: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; DAAs, direct-acting antivirals; EHC, extrahepatic cancer; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; HCV; HR, hazard ratio; Hepatocellular carcinoma; LSM, liver stiffness measurement; Liver failure; MACEs, major adverse cardiovascular events; PHT, portal hypertension; Portal hypertension; SMR, standardised mortality ratio; SVR; SVR, sustained virological response; surveillance.
© 2019 The Author(s).
Figures
Similar articles
-
Risk of hepatocellular carcinoma after HCV eradication: Determining the role of portal hypertension by measuring spleen stiffness.JHEP Rep. 2021 Apr 14;3(3):100289. doi: 10.1016/j.jhepr.2021.100289. eCollection 2021 Jun. JHEP Rep. 2021. PMID: 34095798 Free PMC article.
-
Impact of hepatitis C virus genotype-4 eradication following direct acting antivirals on liver stiffness measurement.Hepat Med. 2017 Oct 6;9:45-53. doi: 10.2147/HMER.S142600. eCollection 2017. Hepat Med. 2017. PMID: 29062242 Free PMC article.
-
Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals.J Hepatol. 2020 Mar;72(3):472-480. doi: 10.1016/j.jhep.2019.10.005. Epub 2019 Oct 17. J Hepatol. 2020. PMID: 31629779
-
Reversion of disease manifestations after HCV eradication.J Hepatol. 2016 Oct;65(1 Suppl):S95-S108. doi: 10.1016/j.jhep.2016.07.039. J Hepatol. 2016. PMID: 27641991 Review.
-
Elastography as a predictor of liver cirrhosis complications after hepatitis C virus eradication in the era of direct-acting antivirals.World J Hepatol. 2021 Nov 27;13(11):1663-1676. doi: 10.4254/wjh.v13.i11.1663. World J Hepatol. 2021. PMID: 34904036 Free PMC article. Review.
Cited by
-
Physiomimetic In Vitro Human Models for Viral Infection in the Liver.Semin Liver Dis. 2023 Feb;43(1):31-49. doi: 10.1055/a-1981-5944. Epub 2022 Nov 19. Semin Liver Dis. 2023. PMID: 36402129 Free PMC article. Review.
-
Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control.J Clin Med. 2021 Jan 19;10(2):353. doi: 10.3390/jcm10020353. J Clin Med. 2021. PMID: 33477752 Free PMC article. Review.
-
After the Storm: Persistent Molecular Alterations Following HCV Cure.Int J Mol Sci. 2024 Jun 27;25(13):7073. doi: 10.3390/ijms25137073. Int J Mol Sci. 2024. PMID: 39000179 Free PMC article. Review.
-
Liver Disease Outcomes after Sustained Virological Response in Patients with Chronic Hepatitis C Infection Treated with Generic Direct-Acting Antivirals.Am J Trop Med Hyg. 2022 Feb 28;106(5):1522-33. doi: 10.4269/ajtmh.21-0918. Online ahead of print. Am J Trop Med Hyg. 2022. PMID: 35226870 Free PMC article.
-
The changing face of hepatology.JHEP Rep. 2019 Dec 30;1(6):415-417. doi: 10.1016/j.jhepr.2019.11.003. eCollection 2019 Dec. JHEP Rep. 2019. PMID: 32039392 Free PMC article. No abstract available.
References
-
- Pawlotsky J.-M., Negro F., Aghemo A., Berenguer M., Dalgard O., Dusheiko G. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. - PubMed
-
- Ioannou G.N., Feld J.J. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection? Gastroenterology. 2019;156:446–460.e2. - PubMed
-
- Terrault N.A., Hassanein T.I. Management of the patient with SVR. J Hepatol. 2016;65:S120–S129. - PubMed
-
- Trinchet J.C., Bourcier V., Chaffaut C., Ait Ahmed M., Allam S., Marcellin P. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort) Hepatology. 2015;62:737–750. - PubMed
-
- Jepsen P., Vilstrup H., Andersen P.K. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology. 2015;62:292–302. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

