Prenatal sonographic features can accurately determine parental origin in triploid pregnancies
- PMID: 32039494
- PMCID: PMC7317806
- DOI: 10.1002/pd.5666
Prenatal sonographic features can accurately determine parental origin in triploid pregnancies
Abstract
Objective: To describe the prenatal sonographic features and maternal biochemical markers in triploid pregnancies and to assess whether prenatal phenotype can determine genetic origin.
Methods: We performed a retrospective multicenter cohort study that included all triploid pregnancies diagnosed between 2000 and 2018 in two Fetal Medicine Units in Amsterdam. Fetal growth, presence of structural anomalies, extra-fetal anomalies, and maternal biochemical markers were retrieved. Asymmetrical intrauterine growth restriction was diagnosed when the head-to-abdominal circumference (HC/AC) ratio was >95th centile. Parental origin was analyzed via molecular genotyping in 46 cases (38.3%).
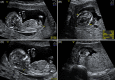
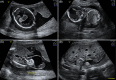
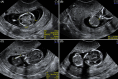
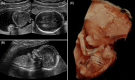
Results: One hundred and twenty triploid pregnancies were identified, of which 86 cases (71.6%) were detected before 18 weeks of gestation. Triploidy of maternal origin was found in 32 cases (69.6%) and was associated with asymmetrical growth restriction, a thin placenta, and low pregnancy-associated plasma protein A and free beta-human chorionic gonadotrophin (β-hCG) levels. Triploidy of paternal origin was found in 14 cases (30.4%) and was associated with an increased nuchal translucency, placental molar changes, and a high free β-hCG. Prospective prediction of the parental origin of the triploidy was made in 30 of the 46 cases based on phenotypical ultrasound presentation, and it was correct in all cases.
Conclusion: Asymmetrical growth restriction with severe HC/AC discrepancy is pathognomonic of maternal triploidy. Placental molar changes indicate a paternal triploidy. Moreover, triploidy can present with an abnormal first trimester combined test, with serum levels on the extreme end. When available results of maternal serum markers can support the diagnosis of parental origin of the triploidy, an accurate assessment of the parental origin based on prenatal sonographic features is possible, making DNA analysis redundant.
© 2020 The Authors. Prenatal Diagnosis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Jacobs PA, Angell RR, Buchanan IM, et al. The origin of human triploids. Ann Hum Genet. 1978;42(1):49‐57. - PubMed
-
- Boue J, Bou A, Lazar P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology. 1975;12(1):11‐26. - PubMed
-
- Szulman AE, Philippe E, Boue JG, et al. Human triploidy: association with partial hydatidiform moles and nonmolar conceptuses. Hum Pathol. 1981;12(11):1016‐1021. - PubMed
-
- Doshi N, Surti U, Szulman AE. Morphologic anomalies in triploid liveborn fetuses. Hum Pathol. 1983;14(8):716‐723. - PubMed
-
- Jauniaux E, Brown R, Snijders RJ, et al. Early prenatal diagnosis of triploidy. Am J Obstet Gynecol. 1997;176(3):550‐554. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

