Fast Imaging for Hyperpolarized MR Metabolic Imaging
- PMID: 32039520
- PMCID: PMC7415658
- DOI: 10.1002/jmri.27070
Fast Imaging for Hyperpolarized MR Metabolic Imaging
Abstract
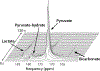
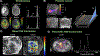
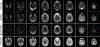
MRI with hyperpolarized carbon-13 agents has created a new type of noninvasive, in vivo metabolic imaging that can be applied in cell, animal, and human studies. The use of 13 C-labeled agents, primarily [1-13 C]pyruvate, enables monitoring of key metabolic pathways with the ability to image substrate and products based on their chemical shift. Over 10 sites worldwide are now performing human studies with this new approach for studies of cancer, heart disease, liver disease, and kidney disease. Hyperpolarized metabolic imaging studies must be performed within several minutes following creation of the hyperpolarized agent due to irreversible decay of the net magnetization back to equilibrium, so fast imaging methods are critical. The imaging methods must include multiple metabolites, separated based on their chemical shift, which are also undergoing rapid metabolic conversion (via label exchange), further exacerbating the challenges of fast imaging. This review describes the state-of-the-art in fast imaging methods for hyperpolarized metabolic imaging. This includes the approach and tradeoffs between three major categories of fast imaging methods-fast spectroscopic imaging, model-based strategies, and metabolite specific imaging-as well additional options of parallel imaging, compressed sensing, tailored RF flip angles, refocused imaging methods, and calibration methods that can improve the scan coverage, speed, signal-to-noise ratio (SNR), resolution, and/or robustness of these studies. To date, these approaches have produced extremely promising initial human imaging results. Improvements to fast hyperpolarized metabolic imaging methods will provide better coverage, SNR, resolution, and reproducibility for future human imaging studies. LEVEL OF EVIDENCE: 5 TECHNICAL EFFICACY STAGE: 1.
Keywords: chemical shift encoding; hyperpolarized carbon-13; metabolic imaging; metabolite-specific imaging; real-time calibration; spectroscopic imaging.
© 2020 International Society for Magnetic Resonance in Medicine.
Figures





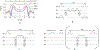


References
-
- Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 2011;13(2):81–97. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

