Impairment of cyclopean surface processing by disparity-defined masking stimuli
- PMID: 32040160
- PMCID: PMC7331773
- DOI: 10.1167/jov.20.2.1
Impairment of cyclopean surface processing by disparity-defined masking stimuli
Abstract
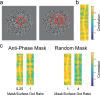
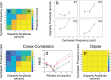
Binocular disparity signals allow for the estimation of three-dimensional shape, even in the absence of monocular depth cues. The perception of such disparity-defined form depends, however, on the linkage of multiple disparity measurements over space. Performance limitations in cyclopean tasks thus inform us about errors arising in disparity measurement and difficulties in the linkage of such measurements. We used a cyclopean orientation discrimination task to examine the perception of disparity-defined form. Participants were presented with random-dot sinusoidal modulations in depth and asked to report whether they were clockwise or counter-clockwise rotated. To assess the effect of different noise structures on measurement and linkage processes, task performance was measured in the presence of binocular, random-dot masks, structured as either antiphase depth sinusoids, or as random distributions of dots in depth. For a fixed number of surface dots, the ratio of mask-to-surface dots was varied to obtain thresholds for orientation discrimination. Antiphase masks were found to be more effective than random depth masks, requiring a lower mask-to-surface dot ratio to inhibit performance. For antiphase masks, performance improved with decreased cyclopean frequency, increased disparity amplitude, and/or an increase in the total number of stimulus dots. Although a cross-correlation model of disparity measurement could account for antiphase mask performance, random depth masking effects were consistent with limitations in relative disparity processing. This suggests that performance is noise-limited for antiphase masks and complexity-limited for random masks. We propose that use of differing mask types may prove effective in understanding these distinct forms of impairment.
Conflict of interest statement
Commercial relationships: none.
Figures





Similar articles
-
The orientation bandwidth of cyclopean channels.Vision Res. 2005 Oct;45(21):2780-5. doi: 10.1016/j.visres.2005.06.006. Vision Res. 2005. PMID: 16040077
-
Disparity biasing in depth from monocular occlusions.Vision Res. 2011 Jul 15;51(14):1699-711. doi: 10.1016/j.visres.2011.05.012. Epub 2011 May 27. Vision Res. 2011. PMID: 21651926
-
Selectivity of cyclopean masking for the spatial frequency of binocular disparity modulation.Vision Res. 1994 Mar;34(5):607-20. doi: 10.1016/0042-6989(94)90016-7. Vision Res. 1994. PMID: 8160380
-
The role of monocularly visible regions in depth and surface perception.Vision Res. 2009 Nov;49(22):2666-85. doi: 10.1016/j.visres.2009.06.021. Epub 2009 Jul 3. Vision Res. 2009. PMID: 19577589 Review.
-
The role of the cyclopean eye in vision: sometimes inappropriate, always irrelevant.Vision Res. 2002 Apr;42(9):1157-63. doi: 10.1016/s0042-6989(01)00280-2. Vision Res. 2002. PMID: 11997054 Review.
References
-
- Brainard D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

