Development of a sheep model of atrioventricular block for the application of novel therapies
- PMID: 32040499
- PMCID: PMC7010276
- DOI: 10.1371/journal.pone.0229092
Development of a sheep model of atrioventricular block for the application of novel therapies
Abstract
Introduction: Sheep have been adopted as a pre-clinical large animal for scientific research as they are good models of cardiac anatomy and physiology, and allow for investigation of pathophysiological processes which occur in the large mammalian heart. There is, however, no defined model of atrioventricular block in sheep to allow for pre-clinical assessment of new cardiac treatment options. We therefore aimed to develop an adult sheep model of atrioventricular block with the focus on future novel applications.
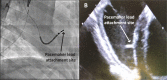
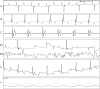
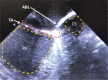
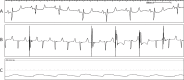
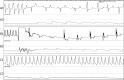
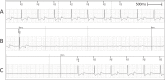
Methods and results: We utilized six sheep to undergo two procedures each. The first procedure involved implantation of a single chamber pacemaker into the right ventricular apex, for baseline assessment over four weeks. The second procedure involved creating atrioventricular block by radiofrequency ablation of the His bundle, before holding for a further four weeks. Interrogation of pacemakers and electrocardiograms determined the persistence of atrioventricular block during the follow up period. Pacemakers were inserted, and atrioventricular block created in 6 animals using a conventional approach. One animal died following ablation of the His bundle, due to procedural complications. Four unablated sheep were assessed for baseline data over four weeks and showed 5.53 ± 1.28% pacing reliance. Five sheep were assessed over four weeks following His bundle ablation and showed continuous (98.89 ± 0.81%) ventricular pacing attributable to persistent atrioventricular block, with no major complications.
Conclusion: We have successfully developed, characterized and validated a large animal model of atrioventricular block that is stable and technically feasible in adult sheep. This model will allow for the advancement of novel therapies, including the development of cell and gene-based therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Plotnikov AN, Sosunov EA, Qu J, Shlapakova IN, Anyukhovsky EP, Liu L, et al. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109(4):506–12. 10.1161/01.CIR.0000114527.10764.CC - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

