Herpes simplex virus encephalitis of childhood: inborn errors of central nervous system cell-intrinsic immunity
- PMID: 32040615
- PMCID: PMC7739442
- DOI: 10.1007/s00439-020-02127-5
Herpes simplex virus encephalitis of childhood: inborn errors of central nervous system cell-intrinsic immunity
Abstract
Herpes simplex virus 1 (HSV-1) encephalitis (HSE) is the most common sporadic viral encephalitis in Western countries. Over the last 15 years, human genetic and immunological studies have provided proof-of-principle that childhood HSE can result from inborn errors of central nervous system (CNS)-specific, cell-intrinsic immunity to HSV-1. HSE-causing mutations of eight genes disrupt known (TLR3-dependent IFN-α/β immunity) and novel (dependent on DBR1 or snoRNA31) antiviral mechanisms. Monogenic inborn errors confer susceptibility to forebrain (TLR3-IFN or snoRNA31) or brainstem (DBR1) HSE. Most of these disorders display incomplete clinical penetrance, with the possible exception of DBR1 deficiency. They account for a small, but non-negligible proportion of cases (about 7%). These findings pave the way for the gradual definition of the genetic and immunological architecture of childhood HSE, with both biological and clinical implications.
Figures
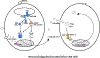
References
-
- Abel L, Plancoulaine S, Jouanguy E, Zhang SY, Mahfoufi N, Nicolas N, Sancho-Shimizu V, Alcais A, Guo Y, Cardon A, Boucherit S, Obach D, Clozel T, Lorenzo L, Amsallem D, Berquin P, Blanc T, Bost-Bru C, Chabrier S, Chabrol B, Cheuret E, Dulac O, Evrard P, Heron B, Lazaro L, Mancini J, Pedespan JM, Rivier F, Vallee L, Lebon P, Rozenberg F, Casanova JL, Tardieu M (2010) Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J Pediatr 157: 623–9, 10.1016/j.jpeds.2010.04.020 - DOI - PubMed
-
- Andersen LL, Mork N, Reinert LS, Kofod-Olsen E, Narita R, Jorgensen SE, Skipper KA, Honing K, Gad HH, Ostergaard L, Orntoft TF, Hornung V, Paludan SR, Mikkelsen JG, Fujita T, Christiansen M, Hartmann R, Mogensen TH (2015) Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J Exp Med 212: 1371–9. doi: 10.1084/jem.20142274 - DOI - PMC - PubMed
-
- Arenas J, Hurwitz J (1987) Purification of a RNA debranching activity from HeLa cells. J Biol Chem 262: 4274–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

