Dibasic Derivatives of Phenylcarbamic Acid as Prospective Antibacterial Agents Interacting with Cytoplasmic Membrane
- PMID: 32041117
- PMCID: PMC7168207
- DOI: 10.3390/antibiotics9020064
Dibasic Derivatives of Phenylcarbamic Acid as Prospective Antibacterial Agents Interacting with Cytoplasmic Membrane
Abstract
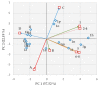
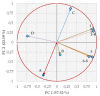
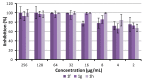
1-[2-[({[2-/3-(Alkoxy)phenyl]amino}carbonyl)oxy]-3-(dipropylammonio)propyl]pyrrolidinium/azepan- ium oxalates or dichlorides (alkoxy = butoxy to heptyloxy) were recently described as very promising antimycobacterial agents. These compounds were tested in vitro against Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212 (reference and control strains), three methicillin-resistant isolates of S. aureus, and three isolates of vancomycin-resistant E. faecalis. 1-[3-(Dipropylammonio)-2-({[3-(pentyloxy-/hexyloxy-/heptyloxy)phenyl]carbamoyl}oxy)propyl]pyrrolidinium dichlorides showed high activity against staphylococci and enterococci comparable with or higher than that of used controls (clinically used antibiotics and antiseptics). The screening of the cytotoxicity of the compounds as well as the used controls was performed using human monocytic leukemia cells. IC50 values of the most effective compounds ranged from ca. 3.5 to 6.3 µM, thus, it can be stated that the antimicrobial effect is closely connected with their cytotoxicity. The antibacterial activity is based on the surface activity of the compounds that are influenced by the length of their alkoxy side chain, the size of the azacyclic system, and hydro-lipophilic properties, as proven by in vitro experiments and chemometric principal component analyses. Synergistic studies showed the increased activity of oxacillin, gentamicin, and vancomycin, which could be explained by the direct activity of the compounds against the bacterial cell wall. All these compounds demonstrate excellent antibiofilm activity, when they inhibit and disrupt the biofilm of S. aureus in concentrations close to minimum inhibitory concentrations against planktonic cells. Expected interactions of the compounds with the cytoplasmic membrane are proven by in vitro crystal violet uptake assays.
Keywords: antibacterial; antibiofilm activity; carbamate; structure–activity relationships; synergy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Dibasic Derivatives of Phenylcarbamic Acid against Mycobacterial Strains: Old Drugs and New Tricks?Molecules. 2018 Sep 28;23(10):2493. doi: 10.3390/molecules23102493. Molecules. 2018. PMID: 30274224 Free PMC article.
-
Insight into antimicrobial activity of substituted phenylcarbamoyloxypiperazinylpropanols.Bioorg Chem. 2020 Sep;102:104060. doi: 10.1016/j.bioorg.2020.104060. Epub 2020 Jun 30. Bioorg Chem. 2020. PMID: 32663668
-
In vitro activity of salicylamide derivatives against vancomycin-resistant enterococci.Bioorg Med Chem Lett. 2018 Jul 1;28(12):2184-2188. doi: 10.1016/j.bmcl.2018.05.011. Epub 2018 May 14. Bioorg Med Chem Lett. 2018. PMID: 29773506
-
Antistaphylococcal Activities and ADME-Related Properties of Chlorinated Arylcarbamoylnaphthalenylcarbamates.Pharmaceuticals (Basel). 2022 Jun 5;15(6):715. doi: 10.3390/ph15060715. Pharmaceuticals (Basel). 2022. PMID: 35745634 Free PMC article.
-
Design, Synthesis and Antimicrobial Properties of New Tetracyclic Quinobenzothiazine Derivatives.Int J Mol Sci. 2022 Dec 1;23(23):15078. doi: 10.3390/ijms232315078. Int J Mol Sci. 2022. PMID: 36499402 Free PMC article.
Cited by
-
New Unnatural Gallotannins: A Way toward Green Antioxidants, Antimicrobials and Antibiofilm Agents.Antioxidants (Basel). 2021 Aug 13;10(8):1288. doi: 10.3390/antiox10081288. Antioxidants (Basel). 2021. PMID: 34439536 Free PMC article.
References
-
- Chattopadhyay D., Das S.K., Patra A.R., Bhattacharya S.K. Non-Antibiotics—An alternative for microbial resistance: Scope and hope. In: Ahmad I., Aqil F., editors. New Strategies Combating Bacterial Infection. Wiley-VCH; Weinhiem, Germany: 2009. pp. 89–125.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

