Synthesis of Functionalized Cannabilactones
- PMID: 32041131
- PMCID: PMC7037859
- DOI: 10.3390/molecules25030684
Synthesis of Functionalized Cannabilactones
Abstract
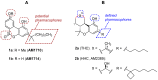
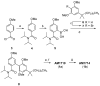
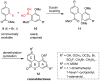
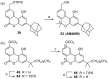
A new approach to synthesize cannabilactones using Suzuki cross-coupling reaction followed by one-step demethylation-cyclization is presented. The two key cannabilactone prototypes AM1710 and AM1714 were obtained selectively in high overall yields and in a lesser number of synthetic steps when compared to our earlier synthesis. The new approach expedited the synthesis of cannabilactone analogs with structural modifications at the four potential pharmacophoric regions.
Keywords: CB2 selective ligands; Suzuki cross-coupling; cannabilactones; cannabinoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cannabilactones: a novel class of CB2 selective agonists with peripheral analgesic activity.J Med Chem. 2007 Dec 27;50(26):6493-500. doi: 10.1021/jm070441u. Epub 2007 Nov 27. J Med Chem. 2007. PMID: 18038967
-
Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery.Mol Pain. 2014 Apr 18;10:27. doi: 10.1186/1744-8069-10-27. Mol Pain. 2014. PMID: 24742127 Free PMC article.
-
Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels.Pain. 2012 May;153(5):1091-1106. doi: 10.1016/j.pain.2012.02.015. Epub 2012 Mar 17. Pain. 2012. PMID: 22425445 Free PMC article.
-
Cannabinoids, endogenous ligands and synthetic analogs.Curr Opin Chem Biol. 1999 Aug;3(4):418-25. doi: 10.1016/S1367-5931(99)80062-3. Curr Opin Chem Biol. 1999. PMID: 10419842 Review.
-
Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications.Recent Pat CNS Drug Discov. 2009 Jun;4(2):112-36. doi: 10.2174/157488909788453031. Recent Pat CNS Drug Discov. 2009. PMID: 19519560 Review.
Cited by
-
Axially Chiral Cannabinoids: Design, Synthesis, and Cannabinoid Receptor Affinity.J Am Chem Soc. 2023 Jun 28;145(25):13581-13591. doi: 10.1021/jacs.3c00129. Epub 2023 Jun 14. J Am Chem Soc. 2023. PMID: 37314891 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

