CB1 Activity Drives the Selection of Navigational Strategies: A Behavioral and c-Fos Immunoreactivity Study
- PMID: 32041135
- PMCID: PMC7036945
- DOI: 10.3390/ijms21031072
CB1 Activity Drives the Selection of Navigational Strategies: A Behavioral and c-Fos Immunoreactivity Study
Abstract
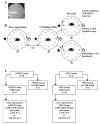
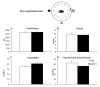
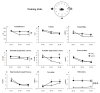
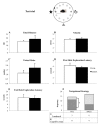
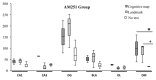
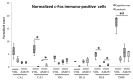
To promote efficient explorative behaviors, subjects adaptively select spatial navigational strategies based on landmarks or a cognitive map. The hippocampus works alone or in conjunction with the dorsal striatum, both representing the neuronal underpinnings of the navigational strategies organized on the basis of different systems of spatial coordinate integration. The high expression of cannabinoid type 1 (CB1) receptors in structures related to spatial learning-such as the hippocampus, dorsal striatum and amygdala-renders the endocannabinoid system a critical target to study the balance between landmark- and cognitive map-based navigational strategies. In the present study, mice treated with the CB1-inverse agonist/antagonist AM251 or vehicle were trained on a Circular Hole Board, a task that could be solved through either navigational strategy. At the end of the behavioral testing, c-Fos immunoreactivity was evaluated in specific nuclei of the hippocampus, dorsal striatum and amygdala. AM251 treatment impaired spatial learning and modified the pattern of the performed navigational strategies as well as the c-Fos immunoreactivity in the hippocampus, dorsal striatum and amygdala. The present findings shed light on the involvement of CB1 receptors as part of the selection system of the navigational strategies implemented to efficiently solve the spatial problem.
Keywords: AM251; Circular Hole Board; amygdala; dorsal striatum; endocannabinoid system; hippocampus; mice; spatial learning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142.Eur Neuropsychopharmacol. 2010 Feb;20(2):112-22. doi: 10.1016/j.euroneuro.2009.11.002. Epub 2009 Dec 16. Eur Neuropsychopharmacol. 2010. PMID: 20015619 Free PMC article.
-
Interaction between hippocampal serotonin and cannabinoid systems in reactivity to spatial and object novelty detection.Behav Brain Res. 2017 Jan 15;317:272-278. doi: 10.1016/j.bbr.2016.09.059. Epub 2016 Sep 26. Behav Brain Res. 2017. PMID: 27686024
-
Systemic or intra-amygdala infusion of an endocannabinoid CB1 receptor antagonist AM251 blocked propofol-induced anterograde amnesia.Neurosci Lett. 2015 Jan 1;584:287-91. doi: 10.1016/j.neulet.2014.11.001. Epub 2014 Nov 6. Neurosci Lett. 2015. PMID: 25445359
-
Differences in spontaneously avoiding or approaching mice reflect differences in CB1-mediated signaling of dorsal striatal transmission.PLoS One. 2012;7(3):e33260. doi: 10.1371/journal.pone.0033260. Epub 2012 Mar 8. PLoS One. 2012. PMID: 22413007 Free PMC article.
-
Sexually-dimorphic alterations in cannabinoid receptor density depend upon prenatal/early postnatal history.Neurotoxicol Teratol. 2016 Nov-Dec;58:31-39. doi: 10.1016/j.ntt.2016.09.004. Epub 2016 Sep 12. Neurotoxicol Teratol. 2016. PMID: 27634313 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Synaptic Plasticity 2.0: Dynamic Changes in Neurons Functions, Physiological and Pathological Process.Int J Mol Sci. 2023 Aug 11;24(16):12685. doi: 10.3390/ijms241612685. Int J Mol Sci. 2023. PMID: 37628862 Free PMC article.
-
Molecular Mechanisms of Synaptic Plasticity: Dynamic Changes in Neuron Functions.Int J Mol Sci. 2023 Aug 8;24(16):12567. doi: 10.3390/ijms241612567. Int J Mol Sci. 2023. PMID: 37628751 Free PMC article.
-
Peripheral CB1 receptor blockade acts as a memory enhancer through a noradrenergic mechanism.Neuropsychopharmacology. 2023 Jan;48(2):341-350. doi: 10.1038/s41386-022-01436-9. Epub 2022 Sep 10. Neuropsychopharmacology. 2023. PMID: 36088492 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

