An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients
- PMID: 32041178
- PMCID: PMC7036826
- DOI: 10.3390/ijms21031074
An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients
Abstract
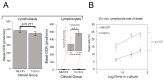
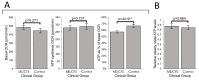
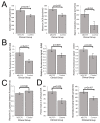
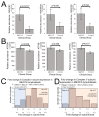
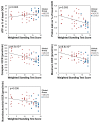
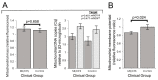
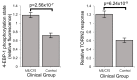
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is an enigmatic condition characterized by exacerbation of symptoms after exertion (post-exertional malaise or "PEM"), and by fatigue whose severity and associated requirement for rest are excessive and disproportionate to the fatigue-inducing activity. There is no definitive molecular marker or known underlying pathological mechanism for the condition. Increasing evidence for aberrant energy metabolism suggests a role for mitochondrial dysfunction in ME/CFS. Our objective was therefore to measure mitochondrial function and cellular stress sensing in actively metabolizing patient blood cells. We immortalized lymphoblasts isolated from 51 ME/CFS patients diagnosed according to the Canadian Consensus Criteria and an age- and gender-matched control group. Parameters of mitochondrial function and energy stress sensing were assessed by Seahorse extracellular flux analysis, proteomics, and an array of additional biochemical assays. As a proportion of the basal oxygen consumption rate (OCR), the rate of ATP synthesis by Complex V was significantly reduced in ME/CFS lymphoblasts, while significant elevations were observed in Complex I OCR, maximum OCR, spare respiratory capacity, nonmitochondrial OCR and "proton leak" as a proportion of the basal OCR. This was accompanied by a reduction of mitochondrial membrane potential, chronically hyperactivated TOR Complex I stress signaling and upregulated expression of mitochondrial respiratory complexes, fatty acid transporters, and enzymes of the β-oxidation and TCA cycles. By contrast, mitochondrial mass and genome copy number, as well as glycolytic rates and steady state ATP levels were unchanged. Our results suggest a model in which ME/CFS lymphoblasts have a Complex V defect accompanied by compensatory upregulation of their respiratory capacity that includes the mitochondrial respiratory complexes, membrane transporters and enzymes involved in fatty acid β-oxidation. This homeostatically returns ATP synthesis and steady state levels to "normal" in the resting cells, but may leave them unable to adequately respond to acute increases in energy demand as the relevant homeostatic pathways are already activated.
Keywords: Complex V; TORC1; chronic fatigue syndrome; mitochondria; myalgic encephalomyelitis; seahorse respirometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dysregulated Provision of Oxidisable Substrates to the Mitochondria in ME/CFS Lymphoblasts.Int J Mol Sci. 2021 Feb 19;22(4):2046. doi: 10.3390/ijms22042046. Int J Mol Sci. 2021. PMID: 33669532 Free PMC article.
-
Extracellular vesicle proteomics uncovers energy metabolism, complement system, and endoplasmic reticulum stress response dysregulation postexercise in males with myalgic encephalomyelitis/chronic fatigue syndrome.Clin Transl Med. 2025 May;15(5):e70346. doi: 10.1002/ctm2.70346. Clin Transl Med. 2025. PMID: 40465195 Free PMC article.
-
Cell-Based Blood Biomarkers for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Int J Mol Sci. 2020 Feb 8;21(3):1142. doi: 10.3390/ijms21031142. Int J Mol Sci. 2020. PMID: 32046336 Free PMC article.
-
Key Pathophysiological Role of Skeletal Muscle Disturbance in Post COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Accumulated Evidence.J Cachexia Sarcopenia Muscle. 2025 Feb;16(1):e13669. doi: 10.1002/jcsm.13669. J Cachexia Sarcopenia Muscle. 2025. PMID: 39727052 Free PMC article. Review.
-
Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an evidence-based approach to diagnosis and management by clinicians.Rev Environ Health. 2015;30(4):223-49. doi: 10.1515/reveh-2015-0026. Rev Environ Health. 2015. PMID: 26613325 Review.
Cited by
-
Surveying the Metabolic and Dysfunctional Profiles of T Cells and NK Cells in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Int J Mol Sci. 2023 Jul 26;24(15):11937. doi: 10.3390/ijms241511937. Int J Mol Sci. 2023. PMID: 37569313 Free PMC article. Review.
-
Altered Metabolic Phenotype of Immune Cells in a Spontaneous Autoimmune Uveitis Model.Front Immunol. 2021 Jul 27;12:601619. doi: 10.3389/fimmu.2021.601619. eCollection 2021. Front Immunol. 2021. PMID: 34385998 Free PMC article.
-
Bioenergetic and Proteomic Profiling of Immune Cells in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients: An Exploratory Study.Biomolecules. 2021 Jun 29;11(7):961. doi: 10.3390/biom11070961. Biomolecules. 2021. PMID: 34209852 Free PMC article.
-
Core features and inherent diversity of post-acute infection syndromes.Front Immunol. 2025 Jun 3;16:1509131. doi: 10.3389/fimmu.2025.1509131. eCollection 2025. Front Immunol. 2025. PMID: 40529374 Free PMC article. Review.
-
Potential Implications of Mammalian Transient Receptor Potential Melastatin 7 in the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Review.Int J Environ Res Public Health. 2021 Oct 12;18(20):10708. doi: 10.3390/ijerph182010708. Int J Environ Res Public Health. 2021. PMID: 34682454 Free PMC article. Review.
References
-
- Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T., Staines D., Powles A.C., Speight N., Vallings R., et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. - DOI - PMC - PubMed
-
- O’Neill H.M., Maarbjerg S.J., Crane J.D., Jeppesen J., Jorgensen S.B., Schertzer J.D., Shyroka O., Kiens B., van Denderen B.J., Tarnopolsky M.A., et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Natl. Acad. Sci. USA. 2011;108:16092–16097. doi: 10.1073/pnas.1105062108. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

