Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells
- PMID: 32041341
- PMCID: PMC7071342
- DOI: 10.3390/nu12020425
Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells
Abstract
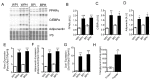
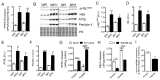
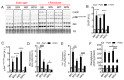
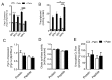
Deregulation of lipid metabolism and insulin function in muscle and adipose tissue are hallmarks of systemic insulin resistance, which can progress to type 2 diabetes. While previous studies suggested that milk proteins influence systemic glucose homeostasis and insulin function, it remains unclear whether bioactive peptides generated from whey alter lipid metabolism and its accumulation in muscle and adipose tissue. Therefore, we incubated murine 3T3-L1 preadipocytes and C2C12 myotubes with a whey peptide mixture produced through pepsin-pancreatin digestion, mimicking peptides generated in the gut from whey protein hydrolysis, and examined its effect on indicators of lipid metabolism and insulin sensitivity. Whey peptides, particularly those derived from bovine serum albumin (BSA), promoted 3T3-L1 adipocyte differentiation and triacylglycerol (TG) accumulation in accordance with peroxisome proliferator-activated receptor γ (PPARγ) upregulation. Whey/BSA peptides also increased lipolysis and mitochondrial fat oxidation in adipocytes, which was associated with the upregulation of peroxisome proliferator-activated receptor δ (PPARδ). In C2C12 myotubes, whey but not BSA peptides ameliorated palmitate-induced insulin resistance, which was associated with reduced inflammation and diacylglycerol accumulation, and increased sequestration of fatty acids in the TG pool. Taken together, our study suggests that whey peptides generated via pepsin-pancreatin digestion profoundly alter lipid metabolism and accumulation in adipocytes and skeletal myotubes.
Keywords: PPAR; adipocytes; differentiation; insulin resistance; lipolysis; lipotoxicity; metabolism; mitochondria; myocytes; whey peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Substance P decreases fat storage and increases adipocytokine production in 3T3-L1 adipocytes.Am J Physiol Gastrointest Liver Physiol. 2013 Feb 15;304(4):G420-7. doi: 10.1152/ajpgi.00162.2012. Epub 2012 Dec 20. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23257919
-
Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3T3-L1 cells.Nutr Res. 2014 Aug;34(8):723-31. doi: 10.1016/j.nutres.2014.07.015. Epub 2014 Jul 30. Nutr Res. 2014. PMID: 25174657
-
Rosiglitazone ameliorates skeletal muscle insulin resistance by decreasing free fatty acids release from adipocytes.Biochem Biophys Res Commun. 2020 Dec 17;533(4):1122-1128. doi: 10.1016/j.bbrc.2020.09.144. Epub 2020 Oct 6. Biochem Biophys Res Commun. 2020. PMID: 33036752
-
Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1).Public Health Nutr. 2007 Oct;10(10A):1132-7. doi: 10.1017/S1368980007000614. Public Health Nutr. 2007. PMID: 17903321 Review.
-
Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes.Nat Rev Mol Cell Biol. 2008 May;9(5):367-77. doi: 10.1038/nrm2391. Nat Rev Mol Cell Biol. 2008. PMID: 18401346 Free PMC article. Review.
Cited by
-
Whey protein hydrolysates improve high-fat-diet-induced obesity by modulating the brain-peripheral axis of GLP-1 through inhibition of DPP-4 function in mice.Eur J Nutr. 2023 Sep;62(6):2489-2507. doi: 10.1007/s00394-023-03162-4. Epub 2023 May 8. Eur J Nutr. 2023. PMID: 37154934
-
Muscle fat content correlates with postoperative survival of viral-related cirrhosis patients after the TIPS: a retrospective study.Ann Med. 2025 Dec;57(1):2484460. doi: 10.1080/07853890.2025.2484460. Epub 2025 Mar 27. Ann Med. 2025. PMID: 40146662 Free PMC article.
-
Sex difference in human diseases: mechanistic insights and clinical implications.Signal Transduct Target Ther. 2024 Sep 10;9(1):238. doi: 10.1038/s41392-024-01929-7. Signal Transduct Target Ther. 2024. PMID: 39256355 Free PMC article. Review.
-
Anti-obesity peptides from food: Production, evaluation, sources, and commercialization.Compr Rev Food Sci Food Saf. 2025 Mar;24(2):e70158. doi: 10.1111/1541-4337.70158. Compr Rev Food Sci Food Saf. 2025. PMID: 40111015 Free PMC article. Review.
-
Association between the sarcopenia index and abnormal liver function in the adult population in the United States: a cross-sectional study.Front Med (Lausanne). 2023 Nov 21;10:1266253. doi: 10.3389/fmed.2023.1266253. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38076231 Free PMC article.
References
-
- Griffin M.E., Marcucci M.J., Cline G.W., Bell K., Barucci N., Lee D., Goodyear L.J., Kraegen E.W., White M.F., Shulman G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

