Sustained Release of Levobupivacaine, Lidocaine, and Acemetacin from Electrosprayed Microparticles: In Vitro and In Vivo Studies
- PMID: 32041361
- PMCID: PMC7037341
- DOI: 10.3390/ijms21031093
Sustained Release of Levobupivacaine, Lidocaine, and Acemetacin from Electrosprayed Microparticles: In Vitro and In Vivo Studies
Abstract
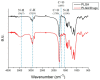
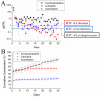
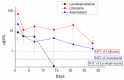
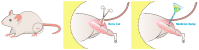
In this study, we explored the release characteristics of analgesics, namely levobupivacaine, lidocaine, and acemetacin, from electrosprayed poly(D,L-lactide-co-glycolide) (PLGA) microparticles. The drug-loaded particles were prepared using electrospraying techniques and evaluated for their morphology, drug release kinetics, and pain relief activity. The morphology of the produced microparticles elucidated by scanning electron microscopy revealed that the optimal parameters for electrospraying were 9 kV, 1 mL/h, and 10 cm for voltage, flow rate, and travel distance, respectively. Fourier-transform infrared spectrometry indicated that the analgesics had been successfully incorporated into the PLGA microparticles. The analgesic-loaded microparticles possessed low toxicity against human fibroblasts and were able to sustainably elute levobupivacaine, lidocaine, and acemetacin in vitro. Furthermore, electrosprayed microparticles were found to release high levels of lidocaine and acemetacin (well over the minimum therapeutic concentrations) and levobupivacaine at the fracture site of rats for more than 28 days and 12 days, respectively. Analgesic-loaded microparticles demonstrated their effectiveness and sustained performance for pain relief in fracture injuries.
Keywords: acemetacin; electrospraying; levobupivacaine; lidocaine; poly(D,L-lactide-co-glycolide); sustained drug release.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control.Polymers (Basel). 2022 Feb 11;14(4):702. doi: 10.3390/polym14040702. Polymers (Basel). 2022. PMID: 35215615 Free PMC article.
-
Preparation, characterization and in vitro cytotoxicity of indomethacin-loaded PLLA/PLGA microparticles using supercritical CO2 technique.Eur J Pharm Biopharm. 2008 Sep;70(1):85-97. doi: 10.1016/j.ejpb.2008.03.011. Epub 2008 Mar 29. Eur J Pharm Biopharm. 2008. PMID: 18495445
-
Sustained Delivery of Analgesic and Antimicrobial Agents to Knee Joint by Direct Injections of Electrosprayed Multipharmaceutical-Loaded Nano/Microparticles.Polymers (Basel). 2018 Aug 9;10(8):890. doi: 10.3390/polym10080890. Polymers (Basel). 2018. PMID: 30960815 Free PMC article.
-
Novel polymeric bioerodable microparticles for prolonged-release intrathecal delivery of analgesic agents for relief of intractable cancer-related pain.J Pharm Sci. 2015 Jul;104(7):2334-44. doi: 10.1002/jps.24497. Epub 2015 May 19. J Pharm Sci. 2015. PMID: 25990226
-
Inhalation of sustained release microparticles for the targeted treatment of respiratory diseases.Drug Deliv Transl Res. 2020 Apr;10(2):339-353. doi: 10.1007/s13346-019-00690-7. Drug Deliv Transl Res. 2020. PMID: 31872342 Review.
Cited by
-
Sustained release local anesthetics for pain management: relevance and formulation approaches.Front Pain Res (Lausanne). 2024 Apr 5;5:1383461. doi: 10.3389/fpain.2024.1383461. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 38645568 Free PMC article. Review.
-
Electrosprayed Core (Cellulose Acetate)-Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery.Pharmaceutics. 2023 Sep 13;15(9):2314. doi: 10.3390/pharmaceutics15092314. Pharmaceutics. 2023. PMID: 37765283 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

