Viscerotropic disease and acute uveitis following yellow fever vaccination: a case report
- PMID: 32041533
- PMCID: PMC7011288
- DOI: 10.1186/s12879-020-4838-x
Viscerotropic disease and acute uveitis following yellow fever vaccination: a case report
Abstract
Background: Yellow fever vaccine exists for over 80 years and is considered to be relatively safe. However, in rare cases it can produce serious neurotropic and viscerotropic complications. We report a case of a patient who presented both viscerotropic and neurological manifestations after yellow fever vaccination.
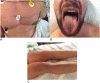
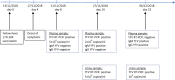
Case presentation: We describe the case of a 37 years old man who developed after the yellow fever vaccination a yellow fever vaccine-associated viscerotropic disease followed by acute uveitis. Prolonged detection of yellow fever RNA in blood and urine was consistent with yellow fever vaccine-associated adverse event. The final outcome was good, although with persistent fatigue over a few months.
Conclusions: Even if the yellow fever vaccine is relatively safe, physicians should be aware of its possible serious adverse effects.
Keywords: Uveitis; Yellow fever vaccine; Yellow fever vaccine-associated viscerotropic disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Yellow fever vaccine usage in the United States and risk of neurotropic and viscerotropic disease: A retrospective cohort study using three healthcare databases.Vaccine. 2022 Jan 31;40(5):742-751. doi: 10.1016/j.vaccine.2021.12.047. Epub 2022 Jan 5. Vaccine. 2022. PMID: 34996642
-
First case of yellow fever vaccine-associated viscerotropic disease (YEL-AVD) in Hong Kong.J Travel Med. 2016 Apr 17;23(4):taw020. doi: 10.1093/jtm/taw020. Print 2016 Apr. J Travel Med. 2016. PMID: 27087559
-
Anterior and Intermediate Uveitis Following Yellow Fever Vaccination with Fractional Dose: Case Reports.Ocul Immunol Inflamm. 2019;27(4):521-523. doi: 10.1080/09273948.2018.1510529. Epub 2018 Aug 28. Ocul Immunol Inflamm. 2019. PMID: 30153765
-
Acute viscerotropic disease following vaccination against yellow fever.Trans R Soc Trop Med Hyg. 2007 Oct;101(10):967-71. doi: 10.1016/j.trstmh.2007.06.013. Epub 2007 Jul 31. Trans R Soc Trop Med Hyg. 2007. PMID: 17669451 Review.
-
Safety of 17D derived yellow fever vaccines.Expert Opin Drug Saf. 2009 Mar;8(2):211-21. doi: 10.1517/14740330902808086. Expert Opin Drug Saf. 2009. PMID: 19309249 Review.
Cited by
-
Activation of an Effective Immune Response after Yellow Fever Vaccination Is Associated with the Genetic Background and Early Response of IFN-γ and CLEC5A.Viruses. 2021 Jan 12;13(1):96. doi: 10.3390/v13010096. Viruses. 2021. PMID: 33445752 Free PMC article.
-
A Yellow Fever Virus 17D Infection and Disease Mouse Model Used to Evaluate a Chimeric Binjari-Yellow Fever Virus Vaccine.Vaccines (Basel). 2020 Jul 9;8(3):368. doi: 10.3390/vaccines8030368. Vaccines (Basel). 2020. PMID: 32660106 Free PMC article.
-
Yellow Fever Vaccine-Related Neurotropic Disease in Brazil Following Immunization with 17DD.Vaccines (Basel). 2023 Feb 15;11(2):445. doi: 10.3390/vaccines11020445. Vaccines (Basel). 2023. PMID: 36851322 Free PMC article.
-
Ocular Inflammation Post-Vaccination.Vaccines (Basel). 2023 Oct 23;11(10):1626. doi: 10.3390/vaccines11101626. Vaccines (Basel). 2023. PMID: 37897028 Free PMC article. Review.
References
-
- Staples JE, Gershman M, Fischer M, Centers for Disease Control and Prevention (CDC) Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2010;59(RR-7):1–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical