Transcriptional profiling and physiological roles of Aedes aegypti spermathecal-related genes
- PMID: 32041546
- PMCID: PMC7011475
- DOI: 10.1186/s12864-020-6543-y
Transcriptional profiling and physiological roles of Aedes aegypti spermathecal-related genes
Abstract
Background: Successful mating of female mosquitoes typically occurs once, with the male sperm being stored in the female spermatheca for every subsequent oviposition event. The female spermatheca is responsible for the maintenance, nourishment, and protection of the male sperm against damage during storage. Aedes aegypti is a major vector of arboviruses, including Yellow Fever, Dengue, Chikungunya, and Zika. Vector control is difficult due to this mosquito high reproductive capacity.
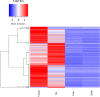
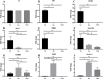
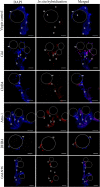
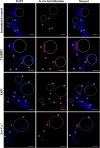
Results: Following comparative RNA-seq analyses of spermathecae obtained from virgin and inseminated females, eight transcripts were selected based on their putative roles in sperm maintenance and survival, including energy metabolism, chitin components, transcriptional regulation, hormonal signaling, enzymatic activity, antimicrobial activity, and ionic homeostasis. In situ RNA hybridization confirmed tissue-specific expression of the eight transcripts. Following RNA interference (RNAi), observed outcomes varied between targeted transcripts, affecting mosquito survival, egg morphology, fecundity, and sperm motility within the spermathecae.
Conclusions: This study identified spermatheca-specific transcripts associated with sperm storage in Ae. aegypti. Using RNAi we characterized the role of eight spermathecal transcripts on various aspects of female fecundity and offspring survival. RNAi-induced knockdown of transcript AeSigP-66,427, coding for a Na+/Ca2+ protein exchanger, specifically interfered with egg production and reduced sperm motility. Our results bring new insights into the molecular basis of sperm storage and identify potential targets for Ae. aegypti control.
Keywords: Ae. Aegypti; Insect reproduction; Sperm; Spermatheca; Transcriptome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Mating and blood-feeding induce transcriptome changes in the spermathecae of the yellow fever mosquito Aedes aegypti.Sci Rep. 2020 Sep 10;10(1):14899. doi: 10.1038/s41598-020-71904-z. Sci Rep. 2020. PMID: 32913240 Free PMC article.
-
Expanded characterization and localization of male seminal fluid proteins within the female reproductive tract of the dengue vector mosquito Aedes aegypti.J Proteomics. 2025 May 15;315:105410. doi: 10.1016/j.jprot.2025.105410. Epub 2025 Feb 19. J Proteomics. 2025. PMID: 39984034
-
Morphological and morphometrical assessment of spermathecae of Aedes aegypti females.Mem Inst Oswaldo Cruz. 2012 Sep;107(6):705-12. doi: 10.1590/s0074-02762012000600001. Mem Inst Oswaldo Cruz. 2012. PMID: 22990957
-
Recent advances in our understanding of pentastomid reproductive biology.Parasitology. 1983 Apr;86 (Pt 4):59-83. doi: 10.1017/s0031182000050848. Parasitology. 1983. PMID: 6346234 Review.
-
The insect spermatheca: an overview.Zoology (Jena). 2017 Apr;121:56-71. doi: 10.1016/j.zool.2016.12.001. Epub 2016 Dec 5. Zoology (Jena). 2017. PMID: 28089345 Review.
Cited by
-
C-Type Lectins Link Immunological and Reproductive Processes in Aedes aegypti.iScience. 2020 Aug 21;23(9):101486. doi: 10.1016/j.isci.2020.101486. Online ahead of print. iScience. 2020. PMID: 32891883 Free PMC article.
-
Seminal fluid proteins induce transcriptome changes in the Aedes aegypti female lower reproductive tract.BMC Genomics. 2021 Dec 15;22(1):896. doi: 10.1186/s12864-021-08201-0. BMC Genomics. 2021. PMID: 34906087 Free PMC article.
-
Gene coexpression network during ontogeny in the yellow fever mosquito, Aedes aegypti.BMC Genomics. 2023 Jun 3;24(1):301. doi: 10.1186/s12864-023-09403-4. BMC Genomics. 2023. PMID: 37270481 Free PMC article.
-
Transcriptomic analysis of the honey bee (Apis mellifera) queen spermathecae reveals genes that may be involved in sperm storage after mating.PLoS One. 2021 Jan 8;16(1):e0244648. doi: 10.1371/journal.pone.0244648. eCollection 2021. PLoS One. 2021. PMID: 33417615 Free PMC article.
-
wMel Wolbachia alters female post-mating behaviors and physiology in the dengue vector mosquito Aedes aegypti.Commun Biol. 2023 Aug 21;6(1):865. doi: 10.1038/s42003-023-05180-8. Commun Biol. 2023. PMID: 37604924 Free PMC article.
References
-
- WHO . Global strategy for dengue prevention and control 2012–2020. Geneva: World Health Organization; 2012.
-
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol. 1990;36(3):165–172. doi: 10.1016/0022-1910(90)90118-Y. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

