Involvement of CXCL12/CXCR4 in the motility of human first-trimester endometrial epithelial cells through an autocrine mechanism by activating PI3K/AKT signaling
- PMID: 32041571
- PMCID: PMC7011269
- DOI: 10.1186/s12884-020-2788-3
Involvement of CXCL12/CXCR4 in the motility of human first-trimester endometrial epithelial cells through an autocrine mechanism by activating PI3K/AKT signaling
Abstract
Background: CXCL12(chemokine ligand 12, CXCL12) and its receptors CXCR4 are widely expressed in maternal-fetal interface and plays an adjust role in materno-fetal dialogue and immune tolerance during early pregnancy. This study aimed to evaluate the role and mechanism of self-derived CXCL12 in modulating the functions of human first-trimester endometrial epithelial cells (EECs) and to identify the potential protein kinase signaling pathways involved in the CXCL12/CXCR4's effect on EECs.
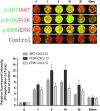
Methods: The expression of CXCL12 and CXCR4 in EECs was measured by using immunohistochemistry, immunofluorescence, real-time polymerase chain reaction and enzyme-linked immunosorbent assay. The effects of EEC-conditioned medium (EEC-CM) and recombinant human CXCL12 (rhCXCL12) on EEC migration and invasion in vitro were evaluated with migration and invasion assays. In-cell western blot analysis was used to examine the phosphorylation of protein kinase B (AKT), extracellular regulated protein kinases (ERKs) and phosphatidylinositol 3-kinase (PI3K) after CXCL12 treatment.
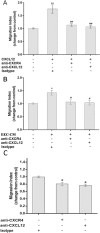
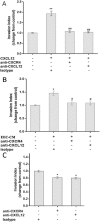
Results: CXCL12 and CXCR4 were both expressed in human first-trimester EECs at the mRNA and protein level. Both EEC-CM and rhCXCL12 significantly increased the migration and invasion of EECs (P < 0.05), which could be blocked by neutralizing antibodies against CXCR4 (P < 0.05) or CXCL12 (P < 0.05), respectively. CXCL12 activated both PI3K/AKT and ERK1/2 signaling and CXCR4 neutralizing antibody effectively reduced CXCL12-induced phosphorylation of AKT and ERK1/2. LY294002, a PI3K-AKT inhibitor, was able to reverse the promotive effect of EEC-CM or rhCXCL12 on EEC migration and invasion.
Conclusions: Human first-trimester EECs promoted their own migration and invasion through the autocrine mechanism with CXCL12/CXCR4 axis involvement by activating PI3K/AKT signaling. This study contributes to a better understanding of the epithelium function mediated by chemokine and chemokine receptor during normal pregnancy.
Keywords: CXCL12/CXCR4; Endometrial epithelial cell (EEC); Maternal-fetal interface; Motility; Phosphorylation.
Conflict of interest statement
Not applicable.
Figures






Similar articles
-
Modulatory effects of trophoblast-secreted CXCL12 on the migration and invasion of human first-trimester decidual epithelial cells are mediated by CXCR4 rather than CXCR7.Reprod Biol Endocrinol. 2018 Mar 2;16(1):17. doi: 10.1186/s12958-018-0333-2. Reprod Biol Endocrinol. 2018. PMID: 29499763 Free PMC article.
-
CXCL12/CXCR4 promotes proliferation, migration, and invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal pathway.J Cell Biochem. 2019 Jun;120(6):9724-9736. doi: 10.1002/jcb.28253. Epub 2018 Dec 23. J Cell Biochem. 2019. PMID: 30582214
-
Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells.J Biol Chem. 2012 Apr 6;287(15):12132-41. doi: 10.1074/jbc.M111.302299. Epub 2012 Feb 15. J Biol Chem. 2012. PMID: 22337890 Free PMC article.
-
Tamoxifen as a modulator of CXCL12-CXCR4-CXCR7 chemokine axis: A breast cancer and glioblastoma view.Cytokine. 2023 Oct;170:156344. doi: 10.1016/j.cyto.2023.156344. Epub 2023 Aug 26. Cytokine. 2023. PMID: 37639844 Review.
-
Is it possible to treat melanoma by intercepting the CXCR4/CXCL12 pathway?Cytokine. 2024 Jul;179:156629. doi: 10.1016/j.cyto.2024.156629. Epub 2024 May 4. Cytokine. 2024. PMID: 38704961 Review.
Cited by
-
Recent Advances in CXCL12/CXCR4 Antagonists and Nano-Based Drug Delivery Systems for Cancer Therapy.Pharmaceutics. 2022 Jul 25;14(8):1541. doi: 10.3390/pharmaceutics14081541. Pharmaceutics. 2022. PMID: 35893797 Free PMC article. Review.
-
MFGE8 mitigates brain injury in a rat model of SAH by maintaining vascular endothelial integrity via TIGβ5/PI3K/CXCL12 signaling.Exp Brain Res. 2021 Jul;239(7):2193-2205. doi: 10.1007/s00221-021-06111-x. Epub 2021 May 15. Exp Brain Res. 2021. PMID: 33991211
-
Endothelial-immune crosstalk contributes to vasculopathy in nonalcoholic fatty liver disease.EMBO Rep. 2022 Jun 7;23(6):e54271. doi: 10.15252/embr.202154271. Epub 2022 Apr 11. EMBO Rep. 2022. PMID: 35403791 Free PMC article.
-
Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids.Elife. 2021 Sep 6;10:e69603. doi: 10.7554/eLife.69603. Elife. 2021. PMID: 34487490 Free PMC article.
-
Role of maternal-fetal immune tolerance in the establishment and maintenance of pregnancy.Chin Med J (Engl). 2024 Jun 20;137(12):1399-1406. doi: 10.1097/CM9.0000000000003114. Epub 2024 May 9. Chin Med J (Engl). 2024. PMID: 38724467 Free PMC article. Review.
References
-
- Ding Jin-Li, Diao Liang-Hui, Yin Tai-Lang, Huang Chun-Yu, Yin Biao, Chen Cong, Zhang Yi, Li Jie, Cheng Yan-Xiang, Zeng Yong, Yang Jing. Aberrant expressions of endometrial Id3 and CTLA-4 are associated with unexplained repeated implantation failure and recurrent miscarriage. American Journal of Reproductive Immunology. 2017;78(2):e12632. doi: 10.1111/aji.12632. - DOI - PubMed
-
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simón C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–824. doi: 10.1016/j.fertnstert.2013.05.004. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

