Effect of tuberculosis screening and retention interventions on early antiretroviral therapy mortality in Botswana: a stepped-wedge cluster randomized trial
- PMID: 32041583
- PMCID: PMC7011529
- DOI: 10.1186/s12916-019-1489-0
Effect of tuberculosis screening and retention interventions on early antiretroviral therapy mortality in Botswana: a stepped-wedge cluster randomized trial
Abstract
Background: Undiagnosed tuberculosis (TB) remains the most common cause of HIV-related mortality. Xpert MTB/RIF (Xpert) is being rolled out globally to improve TB diagnostic capacity. However, previous Xpert impact trials have reported that health system weaknesses blunted impact of this improved diagnostic tool. During phased Xpert rollout in Botswana, we evaluated the impact of a package of interventions comprising (1) additional support for intensified TB case finding (ICF), (2) active tracing for patients missing clinic appointments to support retention, and (3) Xpert replacing sputum-smear microscopy, on early (6-month) antiretroviral therapy (ART) mortality.
Methods: At 22 clinics, ART enrollees > 12 years old were eligible for inclusion in three phases: a retrospective standard of care (SOC), prospective enhanced care (EC), and prospective EC plus Xpert (EC+X) phase. EC and EC+X phases were implemented as a stepped-wedge trial. Participants in the EC phase received SOC plus components 1 (strengthened ICF) and 2 (active tracing) of the intervention package, and participants in the EC+X phase received SOC plus all three intervention package components. Primary and secondary objectives were to compare all-cause 6-month ART mortality between SOC and EC+X and between EC and EC+X phases, respectively. We used adjusted analyses, appropriate for study design, to control for baseline differences in individual-level factors and intra-facility correlation.
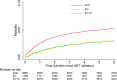
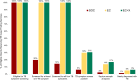
Results: We enrolled 14,963 eligible patients: 8980 in SOC, 1768 in EC, and 4215 in EC+X phases. Median age of ART enrollees was 35 and 64% were female. Median CD4 cell count was lower in SOC than subsequent phases (184/μL in SOC, 246/μL in EC, and 241/μL in EC+X). By 6 months of ART, 461 (5.3%) of SOC, 54 (3.2%) of EC, and 121 (3.0%) of EC+X enrollees had died. Compared with SOC, 6-month mortality was lower in the EC+X phase (adjusted hazard ratio, 0.77; 95% confidence interval, 0.61-0.97, p = 0.029). Compared with EC enrollees, 6-month mortality was similar among EC+X enrollees.
Conclusions: Interventions to strengthen ICF and retention were associated with lower early ART mortality. This new evidence highlights the need to strengthen ICF and retention in many similar settings. Similar to other trials, no additional mortality benefit of replacing sputum-smear microscopy with Xpert was observed.
Trial registration: Retrospectively registered: ClinicalTrials.gov (NCT02538952).
Keywords: Intensified tuberculosis case finding; Mortality; Tuberculosis; Xpert MTB/RIF.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. - DOI - PMC - PubMed
-
- World Health Organisation. Rapid Implementation of the Xpert MTB/RIF diagnostic test. http://whqlibdoc.who.int/publications/2011/9789241501569_eng.pdf. Accessed 10 Aug 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

