HIV-1 acquired drug resistance to integrase inhibitors in a cohort of antiretroviral therapy multi-experienced Mexican patients failing to raltegravir: a cross-sectional study
- PMID: 32041622
- PMCID: PMC7011548
- DOI: 10.1186/s12981-020-0262-y
HIV-1 acquired drug resistance to integrase inhibitors in a cohort of antiretroviral therapy multi-experienced Mexican patients failing to raltegravir: a cross-sectional study
Abstract
Background: In resource-limited settings, multi-experienced HIV infected patients are often prescribed raltegravir for salvage therapy. Patients failing raltegravir-containing regimens require other drugs including other integrase inhibitors. In this context, real-life data about the resistance and cross-resistance pathways between integrase inhibitors is limited. The aim of this study was to investigate integrase resistance pathways in a cohort of Mexican multi-experienced patients failing of a raltegravir-containing salvage regimen.
Methods: Twenty-five plasma samples from subjects failing antiretroviral regimens which included raltegravir were obtained from various healthcare centres from 2009 to 2017 in Mexico. Antiretroviral history and demographics were collected. Samples were processed for integrase resistance genotyping testing by sequencing. The viral sequences were analysed with the Stanford HIV drug resistance database algorithm. Data was analysed with SPSS Statistics software.
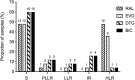
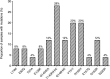
Results: We found a mean viral load of 4.17 log10 c/mL (SD 1.11) at the time of virologic failure. Forty-eight percent of the samples were raltegravir resistant. The Y143R/H/C substitutions were the most prevalent, followed by the N155H, and both Q148H/K and G140S/A in the same proportion. The Q148 + G140 combination was found in (12%) of the samples. Cross-resistance to elvitegravir was found in 83.3% and in 18.2% for both dolutegravir and bictegravir. Thirteen samples (52%) were susceptible to the four integrase strand-transfer inhibitors.
Conclusions: Our findings suggest a high occurrence of resistance and cross-resistance to other integrase inhibitors among multi-experienced subjects failing raltegravir. We found a modestly lower proportion of cross-resistance to dolutegravir than data from clinical trials. Likely this drug could be used for salvage therapy. Explanations for the absence of mutations in half of the samples, other than reduced adherence, should be further investigated. Close surveillance is needed.
Keywords: Cross-resistance; Drug resistance; Integrase inhibitors; Multi-experienced; Raltegravir; Sequencing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Combination of two pathways involved in raltegravir resistance confers dolutegravir resistance.J Antimicrob Chemother. 2015 Oct;70(10):2870-80. doi: 10.1093/jac/dkv197. Epub 2015 Jul 23. J Antimicrob Chemother. 2015. PMID: 26205139
-
Cross-resistance to elvitegravir and dolutegravir in 502 patients failing on raltegravir: a French national study of raltegravir-experienced HIV-1-infected patients.J Antimicrob Chemother. 2015 May;70(5):1507-12. doi: 10.1093/jac/dku535. Epub 2015 Jan 3. J Antimicrob Chemother. 2015. PMID: 25558077
-
New raltegravir resistance pathways induce broad cross-resistance to all currently used integrase inhibitors.J Antimicrob Chemother. 2014 Aug;69(8):2118-22. doi: 10.1093/jac/dku095. Epub 2014 Apr 7. J Antimicrob Chemother. 2014. PMID: 24710029
-
Characterization and structural analysis of HIV-1 integrase conservation.AIDS Rev. 2009 Jan-Mar;11(1):17-29. AIDS Rev. 2009. PMID: 19290031 Review.
-
[Resistance to integrase inhibitors].Enferm Infecc Microbiol Clin. 2008 Nov;26 Suppl 12:40-6. doi: 10.1016/s0213-005x(08)76572-8. Enferm Infecc Microbiol Clin. 2008. PMID: 19572425 Review. Spanish.
Cited by
-
HIV-1 Genetic Diversity and Natural Polymorphisms of the Integrase Gene in Integrase Inhibitor-Naive Patients in Harare, Zimbabwe.AIDS Res Hum Retroviruses. 2021 Dec;37(12):954-961. doi: 10.1089/AID.2021.0084. AIDS Res Hum Retroviruses. 2021. PMID: 34714124 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

