Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up
- PMID: 32041686
- PMCID: PMC7012595
- DOI: 10.7554/eLife.51507
Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up
Abstract
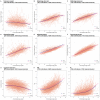
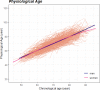
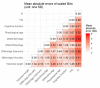
Biological age measurements (BAs) assess aging-related physiological change and predict health risks among individuals of the same chronological age (CA). Multiple BAs have been proposed and are well studied individually but not jointly. We included 845 individuals and 3973 repeated measurements from a Swedish population-based cohort and examined longitudinal trajectories, correlations, and mortality associations of nine BAs across 20 years follow-up. We found the longitudinal growth of functional BAs accelerated around age 70; average levels of BA curves differed by sex across the age span (50-90 years). All BAs were correlated to varying degrees; correlations were mostly explained by CA. Individually, all BAs except for telomere length were associated with mortality risk independently of CA. The largest effects were seen for methylation age estimators (GrimAge) and the frailty index (FI). In joint models, two methylation age estimators (Horvath and GrimAge) and FI remained predictive, suggesting they are complementary in predicting mortality.
Keywords: aging; biological age; correlation; epidemiology; global health; human; longitudinal trajectory; mortality.
Plain language summary
Everyone ages, but how aging affects health varies from person to person. This means that how old someone seems or feels does not always match the number of years they have been alive; in other words, someone’s “biological age” can often differ from their “chronological age”. Scientists are now looking at the physiological changes related to aging to better predict who is at the greatest risk of age-related health problems. Several measurements of biological age have been put forward to capture information about various age-related changes. For example, some measurements look at changes to DNA molecules, while others measure signs of frailty, or deterioration in cognitive or physical abilities. However, to date, most studies into measures of biological age have looked at them individually and less is known about how these physiological changes interact, which is likely to be important. Now, Li et al. have looked at data on nine different measures of biological age in a group of 845 Swedish adults, aged between 50 and 90, that was collected several times over a follow-up period of about 20 years. The dataset also gave details of the individuals’ birth year, sex, height, weight, smoking status, and education. The year of death was also collected from national registers for all individual in the group who had since died. Li et al. found that all nine biological age measures could be used to explain the risk of individuals in the group dying during the follow-up period. In other words, when comparing individuals with the same chronological age in the group under study, the person with a higher biological age measure was more likely to die earlier. The analysis also revealed that biological aging appears to accelerate as individuals approach 70 years old, and that there are noticeable differences in the aging process between men and women. Lastly, when combining all nine biological age measures, some of them worked better than others. Measurements of methylation groups added to DNA (known as DNA methylation age) and frailty (the frailty index) led to improved predictions for an individual’s risk of death. Ultimately, if future studies confirm these results for measures from single individuals, DNA methylation and the frailty index may be used to help identify people who may benefit the most from interventions to prevent age-related health conditions.
© 2020, Li et al.
Conflict of interest statement
XL, AP, YW, PM, CR, DF, NP, JJ, SH No competing interests declared
Figures



Comment in
-
The challenges of estimating biological age.Elife. 2020 Feb 11;9:e54969. doi: 10.7554/eLife.54969. Elife. 2020. PMID: 32041685 Free PMC article.
References
-
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, Caspi A. Eleven telomere, epigenetic clock and Biomarker-Composite Quantifications of Biological Aging: Do They Measure the Same Thing? American Journal of Epidemiology. 2018;187:1220–1230. doi: 10.1093/aje/kwx346. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

