Multiple Low-Reactivity Class B Penicillin-Binding Proteins Are Required for Cephalosporin Resistance in Enterococci
- PMID: 32041714
- PMCID: PMC7179317
- DOI: 10.1128/AAC.02273-19
Multiple Low-Reactivity Class B Penicillin-Binding Proteins Are Required for Cephalosporin Resistance in Enterococci
Abstract
Enterococcus faecalis and Enterococcus faecium are commensals of the gastrointestinal tract of most terrestrial organisms, including humans, and are major causes of health care-associated infections. Such infections are difficult or impossible to treat, as the enterococcal strains responsible are often resistant to multiple antibiotics. One intrinsic resistance trait that is conserved among E. faecalis and E. faecium is cephalosporin resistance, and prior exposure to cephalosporins is one of the most well-known risk factors for acquisition of an enterococcal infection. Cephalosporins inhibit peptidoglycan biosynthesis by acylating the active-site serine of penicillin-binding proteins (PBPs) to prevent the PBPs from catalyzing cross-linking during peptidoglycan synthesis. For decades, a specific PBP (known as Pbp4 or Pbp5) that exhibits low reactivity toward cephalosporins has been thought to be the primary PBP required for cephalosporin resistance. We analyzed other PBPs and report that in both E. faecalis and E. faecium, a second PBP, PbpA(2b), is also required for resistance; notably, the cephalosporin ceftriaxone exhibits a lethal effect on the ΔpbpA mutant. Strikingly, PbpA(2b) exhibits low intrinsic reactivity with cephalosporins in vivo and in vitro Unlike the Δpbp5 mutant, the ΔpbpA mutant exhibits a variety of phenotypic defects in growth kinetics, cell wall integrity, and cellular morphology, indicating that PbpA(2b) and Pbp5(4) are not functionally redundant and that PbpA(2b) plays a more central role in peptidoglycan synthesis. Collectively, our results shift the current understanding of enterococcal cephalosporin resistance and suggest a model in which PbpA(2b) and Pbp5(4) cooperate to coordinately mediate peptidoglycan cross-linking in the presence of cephalosporins.
Keywords: beta-lactam resistance; cephalosporins; enterococci; penicillin-binding proteins; peptidoglycan synthesis.
Copyright © 2020 American Society for Microbiology.
Figures
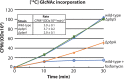
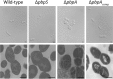



Similar articles
-
CroR Regulates Expression of pbp4(5) to Promote Cephalosporin Resistance in Enterococcus faecalis.mBio. 2022 Aug 30;13(4):e0111922. doi: 10.1128/mbio.01119-22. Epub 2022 Aug 1. mBio. 2022. PMID: 35913163 Free PMC article.
-
Involvement of the Eukaryote-Like Kinase-Phosphatase System and a Protein That Interacts with Penicillin-Binding Protein 5 in Emergence of Cephalosporin Resistance in Cephalosporin-Sensitive Class A Penicillin-Binding Protein Mutants in Enterococcus faecium.mBio. 2016 Apr 5;7(2):e02188-15. doi: 10.1128/mBio.02188-15. mBio. 2016. PMID: 27048803 Free PMC article.
-
The structures of penicillin-binding protein 4 (PBP4) and PBP5 from Enterococci provide structural insights into β-lactam resistance.J Biol Chem. 2018 Nov 30;293(48):18574-18584. doi: 10.1074/jbc.RA118.006052. Epub 2018 Oct 24. J Biol Chem. 2018. PMID: 30355734 Free PMC article.
-
Intrinsic penicillin resistance in enterococci.Microb Drug Resist. 1996 Summer;2(2):209-13. doi: 10.1089/mdr.1996.2.209. Microb Drug Resist. 1996. PMID: 9158761 Review.
-
Antibiotic resistant enterococci-tales of a drug resistance gene trafficker.Int J Med Microbiol. 2013 Aug;303(6-7):360-79. doi: 10.1016/j.ijmm.2013.03.001. Epub 2013 Mar 8. Int J Med Microbiol. 2013. PMID: 23602510 Review.
Cited by
-
Use of an Interspecies Chimeric Receptor for Inducible Gene Expression Reveals that Metabolic Flux through the Peptidoglycan Biosynthesis Pathway is an Important Driver of Cephalosporin Resistance in Enterococcus faecalis.J Bacteriol. 2022 Apr 19;204(4):e0060221. doi: 10.1128/jb.00602-21. Epub 2022 Mar 8. J Bacteriol. 2022. PMID: 35258319 Free PMC article.
-
Epidemiology of vancomycin-resistant enterococci in the United Arab Emirates: a retrospective analysis of 12 years of national AMR surveillance data.Front Public Health. 2023 Nov 27;11:1275778. doi: 10.3389/fpubh.2023.1275778. eCollection 2023. Front Public Health. 2023. PMID: 38089023 Free PMC article.
-
The HtrA chaperone monitors sortase-assembled pilus biogenesis in Enterococcus faecalis.PLoS Genet. 2024 Aug 5;20(8):e1011071. doi: 10.1371/journal.pgen.1011071. eCollection 2024 Aug. PLoS Genet. 2024. PMID: 39102428 Free PMC article.
-
Lytic bacteriophages facilitate antibiotic sensitization of Enterococcus faecium.Antimicrob Agents Chemother. 2023 May 1;65(5):e00143-21. doi: 10.1128/AAC.00143-21. Epub 2021 Mar 1. Antimicrob Agents Chemother. 2023. PMID: 33649110 Free PMC article.
-
Rapid detection of β-lactamase activity using the rapid Amp NP test.Microbiol Spectr. 2025 Apr;13(4):e0078224. doi: 10.1128/spectrum.00782-24. Epub 2025 Mar 6. Microbiol Spectr. 2025. PMID: 40047444 Free PMC article.
References
-
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, Participating National Healthcare Safety Network Facilities . 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi:10.1086/591861. - DOI - PubMed
-
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi:10.1017/ice.2016.174. - DOI - PMC - PubMed
-
- Kristich CJ, Rice LB, Arias CA. 2014. Enterococcal infection-treatment and antibiotic resistance In Gilmore MS, Clewell DB, Ike Y, Shankar N. (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

