Population Pharmacokinetics of Amikacin Administered Once Daily in Patients with Different Renal Functions
- PMID: 32041715
- PMCID: PMC7179627
- DOI: 10.1128/AAC.02178-19
Population Pharmacokinetics of Amikacin Administered Once Daily in Patients with Different Renal Functions
Abstract
The aim of this work was to evaluate the pharmacokinetics of amikacin in Mexican patients with different renal functions receiving once-daily dosing regimens and the influence of clinical and demographical covariates that may influence the optimization of this antibiotic. A prospective study was performed in a total of 63 patients with at least one determination of amikacin plasma concentration. Population pharmacokinetic (PK) parameters were estimated by nonlinear mixed-effects modeling; validations were performed for dosing recommendation purposes based on PK/pharmacodynamic simulations. The concentration-versus-time data were best described by a one-compartment open model with proportional interindividual variability associated with amikacin clearance (CL) and volume of distribution (V); residual error followed a homoscedastic trend. Creatinine clearance (CLCR) and ideal body weight (IBW) demonstrated significant influence on amikacin CL and V, respectively. The final model [CL (liters/h) = 7.1 × (CLCR/130)0.84 and V (liters) = 20.3 × (IBW/68)2.9] showed a mean prediction error of 0.11 mg/liter (95% confidence interval, -3.34, 3.55) in the validation performed in a different group of patients with similar characteristics. There is a wide variability in amikacin PK parameters in Mexican patients. This leads to inadequate dosing regimens, especially in patients with augmented renal clearance (CLCR of >130 ml/min). Optimization based on the final population PK model in Mexican patients may be useful, since reliability and clinical applicability have been demonstrated in this study.
Keywords: PK/PD modeling; amikacin; augmented renal clearance; population pharmacokinetics.
Copyright © 2020 American Society for Microbiology.
Figures
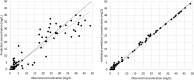
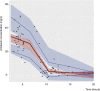

Comment in
-
Urinary Creatinine Clearance and Pharmacokinetics Studies: If We Can Measure It, Why Do We Estimate It?Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00980-20. doi: 10.1128/AAC.00980-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32571825 Free PMC article. No abstract available.
Similar articles
-
Population Pharmacokinetic Study of the Suitability of Standard Dosing Regimens of Amikacin in Critically Ill Patients with Open-Abdomen and Negative-Pressure Wound Therapy.Antimicrob Agents Chemother. 2020 Mar 24;64(4):e02098-19. doi: 10.1128/AAC.02098-19. Print 2020 Mar 24. Antimicrob Agents Chemother. 2020. PMID: 31964795 Free PMC article.
-
Evaluating renal function and age as predictors of amikacin clearance in neonates: model-based analysis and optimal dosing strategies.Br J Clin Pharmacol. 2016 Sep;82(3):793-805. doi: 10.1111/bcp.13016. Epub 2016 Jun 30. Br J Clin Pharmacol. 2016. PMID: 27198625 Free PMC article. Clinical Trial.
-
Influence of Renal Replacement Modalities on Amikacin Population Pharmacokinetics in Critically Ill Patients on Continuous Renal Replacement Therapy.Antimicrob Agents Chemother. 2016 Jul 22;60(8):4901-9. doi: 10.1128/AAC.00828-16. Print 2016 Aug. Antimicrob Agents Chemother. 2016. PMID: 27270279 Free PMC article. Clinical Trial.
-
Amikacin in Critically Ill Patients: A Review of Population Pharmacokinetic Studies.Clin Pharmacokinet. 2017 Feb;56(2):127-138. doi: 10.1007/s40262-016-0428-x. Clin Pharmacokinet. 2017. PMID: 27324191 Review.
-
Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling?Antibiotics (Basel). 2021 Apr 29;10(5):507. doi: 10.3390/antibiotics10050507. Antibiotics (Basel). 2021. PMID: 33946905 Free PMC article. Review.
Cited by
-
Therapeutic Drug Monitoring-Based Population Pharmacokinetics of Amikacin in Patients at a Teaching Hospital.Antibiotics (Basel). 2025 May 22;14(6):531. doi: 10.3390/antibiotics14060531. Antibiotics (Basel). 2025. PMID: 40558121 Free PMC article.
-
Population Pharmacokinetic Analysis of Amikacin for Optimal Pharmacotherapy in Korean Patients with Nontuberculous Mycobacterial Pulmonary Disease.Antibiotics (Basel). 2020 Nov 6;9(11):784. doi: 10.3390/antibiotics9110784. Antibiotics (Basel). 2020. PMID: 33172135 Free PMC article.
-
Failure to predict amikacin elimination in critically ill patients with cancer based on the estimated glomerular filtration rate: applying PBPK approach in a therapeutic drug monitoring study.Eur J Clin Pharmacol. 2023 Jul;79(7):1003-1012. doi: 10.1007/s00228-023-03516-1. Epub 2023 May 31. Eur J Clin Pharmacol. 2023. PMID: 37256410
-
Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis.Pharmaceutics. 2022 Feb 19;14(2):445. doi: 10.3390/pharmaceutics14020445. Pharmaceutics. 2022. PMID: 35214177 Free PMC article. Review.
-
Population Pharmacokinetics of Amikacin in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation.Pharmaceutics. 2022 Jan 26;14(2):289. doi: 10.3390/pharmaceutics14020289. Pharmaceutics. 2022. PMID: 35214022 Free PMC article.
References
-
- Isaksson B, Hanberger H, Maller R, Nilsson LE, Nilsson M. 1990. The postantibiotic effect of amikacin alone and in combination with piperacillin on gram-negative bacteria. Scand J Infect Dis Suppl 74:129–132. - PubMed
-
- de Montmollin E, Bouadma L, Gault N, Mourvillier B, Mariotte E, Chemam S, Massias L, Papy E, Tubach F, Wolff M, Sonneville R. 2014. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med 40:998–1005. doi:10.1007/s00134-014-3276-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

