Synergistic Interactions of Indole-2-Carboxamides and β-Lactam Antibiotics against Mycobacterium abscessus
- PMID: 32041716
- PMCID: PMC7179587
- DOI: 10.1128/AAC.02548-19
Synergistic Interactions of Indole-2-Carboxamides and β-Lactam Antibiotics against Mycobacterium abscessus
Abstract
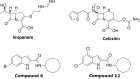
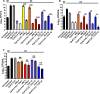
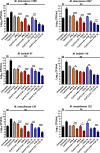
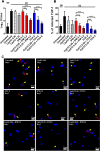
New drugs or therapeutic combinations are urgently needed against Mycobacterium abscessus Previously, we demonstrated the potent activity of indole-2-carboxamides 6 and 12 against M. abscessus We show here that these compounds act synergistically with imipenem and cefoxitin in vitro and increase the bactericidal activity of the β-lactams against M. abscessus In addition, compound 12 also displays synergism with imipenem and cefoxitin within infected macrophages. The clinical potential of these new drug combinations requires further evaluation.
Keywords: MmpL3; Mycobacterium abscessus; drug synergism; indole-2-carboxamide; macrophage; therapeutic activity; β-lactam.
Copyright © 2020 American Society for Microbiology.
Figures
Similar articles
-
Dual β-Lactam Combinations Highly Active against Mycobacterium abscessus Complex In Vitro.mBio. 2019 Feb 12;10(1):e02895-18. doi: 10.1128/mBio.02895-18. mBio. 2019. PMID: 30755518 Free PMC article.
-
Effect of β-lactamase production and β-lactam instability on MIC testing results for Mycobacterium abscessus.J Antimicrob Chemother. 2017 Nov 1;72(11):3070-3078. doi: 10.1093/jac/dkx284. J Antimicrob Chemother. 2017. PMID: 28961987
-
Combined dual β-lactams and diazabicyclooctane β-lactamase inhibitor is highly effective against Mycobacterium abscessus species in vitro.J Glob Antimicrob Resist. 2025 May;42:142-150. doi: 10.1016/j.jgar.2025.02.015. Epub 2025 Feb 25. J Glob Antimicrob Resist. 2025. PMID: 40015478
-
Dual β-lactam therapy to improve treatment outcome in Mycobacterium abscessus disease.Clin Microbiol Infect. 2024 Jun;30(6):738-742. doi: 10.1016/j.cmi.2024.03.019. Epub 2024 Mar 23. Clin Microbiol Infect. 2024. PMID: 38527611 Review.
-
Dual β-lactams for the treatment of Mycobacterium abscessus: a review of the evidence and a call to act against an antibiotic nightmare.J Antimicrob Chemother. 2024 Nov 4;79(11):2731-2741. doi: 10.1093/jac/dkae288. J Antimicrob Chemother. 2024. PMID: 39150384 Free PMC article. Review.
Cited by
-
New therapeutic strategies for Mycobacterium abscessus pulmonary diseases - untapping the mycolic acid pathway.Expert Rev Anti Infect Ther. 2023 Jul-Dec;21(8):813-829. doi: 10.1080/14787210.2023.2224563. Epub 2023 Jun 23. Expert Rev Anti Infect Ther. 2023. PMID: 37314394 Free PMC article. Review.
-
Metabolic state-driven nutrient-based approach to combat bacterial antibiotic resistance.NPJ Antimicrob Resist. 2025 Apr 4;3(1):24. doi: 10.1038/s44259-025-00092-5. NPJ Antimicrob Resist. 2025. PMID: 40185857 Free PMC article. Review.
-
Current Molecular Therapeutic Agents and Drug Candidates for Mycobacterium abscessus.Front Pharmacol. 2021 Aug 30;12:724725. doi: 10.3389/fphar.2021.724725. eCollection 2021. Front Pharmacol. 2021. PMID: 34526902 Free PMC article. Review.
-
Pipeline of anti-Mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities.Med Res Rev. 2021 Jul;41(4):2350-2387. doi: 10.1002/med.21798. Epub 2021 Mar 1. Med Res Rev. 2021. PMID: 33645845 Free PMC article. Review.
-
Clofazimine as a comparator for preclinical efficacy evaluations of experimental therapeutics against pulmonary M. abscessus infection in mice.Tuberculosis (Edinb). 2022 Dec;137:102268. doi: 10.1016/j.tube.2022.102268. Epub 2022 Oct 4. Tuberculosis (Edinb). 2022. PMID: 36228452 Free PMC article.
References
-
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi:10.1164/rccm.200604-571ST. - DOI - PubMed
-
- Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi:10.1136/thoraxjnl-2015-207983. - DOI - PMC - PubMed
-
- Dupont C, Viljoen A, Dubar F, Blaise M, Bernut A, Pawlik A, Bouchier C, Brosch R, Guérardel Y, Lelièvre J, Ballell L, Herrmann J-L, Biot C, Kremer L. 2016. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol Microbiol 101:515–529. doi:10.1111/mmi.13406. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

