The E3 ubiquitin ligase STUB1 attenuates cell senescence by promoting the ubiquitination and degradation of the core circadian regulator BMAL1
- PMID: 32041778
- PMCID: PMC7135990
- DOI: 10.1074/jbc.RA119.011280
The E3 ubiquitin ligase STUB1 attenuates cell senescence by promoting the ubiquitination and degradation of the core circadian regulator BMAL1
Erratum in
-
Correction: The E3 ubiquitin ligase STUB1 attenuates cell senescence by promoting the ubiquitination and degradation of the core circadian regulator BMAL1.J Biol Chem. 2020 Aug 7;295(32):11378. doi: 10.1074/jbc.AAC120.015160. J Biol Chem. 2020. PMID: 32769176 Free PMC article. No abstract available.
Abstract
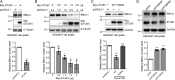
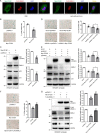
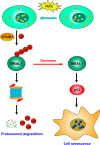
Cell senescence is one of the most important processes determining cell fate and is involved in many pathophysiological conditions, including cancer, neurodegenerative diseases, and other aging-associated diseases. It has recently been discovered that the E3 ubiquitin ligase STIP1 homology and U-box-containing protein 1 (STUB1 or CHIP) is up-regulated during the senescence of human fibroblasts and modulates cell senescence. However, the molecular mechanism underlying STUB1-controlled senescence is not clear. Here, using affinity purification and MS-based analysis, we discovered that STUB1 binds to brain and muscle ARNT-like 1 (BMAL1, also called aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL)). Through biochemical experiments, we confirmed the STUB1-BMAL1 interaction, identified their interaction domains, and revealed that STUB1 overexpression down-regulates BMAL1 protein levels through STUB1's enzymatic activity and that STUB1 knockdown increases BMAL1 levels. Further experiments disclosed that STUB1 enhances BMAL1 degradation, which is abolished upon proteasome inhibition. Moreover, we found that STUB1 promotes the formation of Lys-48-linked polyubiquitin chains on BMAL1, facilitating its proteasomal degradation. Interestingly, we also discovered that oxidative stress promotes STUB1 nuclear translocation and enhances its co-localization with BMAL1. STUB1 expression attenuates hydrogen peroxide-induced cell senescence, indicated by a reduced signal in senescence-associated β-gal staining and decreased protein levels of two cell senescence markers, p53 and p21. BMAL1 knockdown diminishes this effect, and BMAL1 overexpression abolishes STUB1's effect on cell senescence. In summary, the results of our work reveal that the E3 ubiquitin ligase STUB1 ubiquitinates and degrades its substrate BMAL1 and thereby alleviates hydrogen peroxide-induced cell senescence.
Keywords: E3 ubiquitin ligase; STIP1 homology and U-box-containing protein 1 (STUB1); brain and muscle ARNT-like 1 (BMAL1, ARNTL, MOP3); cell cycle regulation; circadian clock; hydrogen peroxide; proteasome; protein degradation; senescence; ubiquitylation (ubiquitination).
© 2020 Ullah et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Ubiquitin-conjugating enzyme UBE2O regulates cellular clock function by promoting the degradation of the transcription factor BMAL1.J Biol Chem. 2018 Jul 20;293(29):11296-11309. doi: 10.1074/jbc.RA117.001432. Epub 2018 Jun 5. J Biol Chem. 2018. PMID: 29871923 Free PMC article.
-
Ubiquitin ligase TRAF2 attenuates the transcriptional activity of the core clock protein BMAL1 and affects the maximal Per1 mRNA level of the circadian clock in cells.FEBS J. 2018 Aug;285(16):2987-3001. doi: 10.1111/febs.14595. Epub 2018 Jul 5. FEBS J. 2018. PMID: 29935055
-
E3 ubiquitin ligase UBR5 modulates circadian rhythm by facilitating the ubiquitination and degradation of the key clock transcription factor BMAL1.Acta Pharmacol Sin. 2024 Sep;45(9):1793-1808. doi: 10.1038/s41401-024-01290-z. Epub 2024 May 13. Acta Pharmacol Sin. 2024. PMID: 38740904 Free PMC article.
-
STUB1/CHIP: New insights in cancer and immunity.Biomed Pharmacother. 2023 Sep;165:115190. doi: 10.1016/j.biopha.2023.115190. Epub 2023 Jul 26. Biomed Pharmacother. 2023. PMID: 37506582 Review.
-
Design Principles Involving Protein Disorder Facilitate Specific Substrate Selection and Degradation by the Ubiquitin-Proteasome System.J Biol Chem. 2016 Mar 25;291(13):6723-31. doi: 10.1074/jbc.R115.692665. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851277 Free PMC article. Review.
Cited by
-
PQBP3 prevents senescence by suppressing PSME3-mediated proteasomal Lamin B1 degradation.EMBO J. 2024 Sep;43(18):3968-3999. doi: 10.1038/s44318-024-00192-4. Epub 2024 Aug 5. EMBO J. 2024. PMID: 39103492 Free PMC article.
-
Chaperone-assisted E3 ligase CHIP: A double agent in cancer.Genes Dis. 2021 Sep 1;9(6):1521-1555. doi: 10.1016/j.gendis.2021.08.003. eCollection 2022 Nov. Genes Dis. 2021. PMID: 36157498 Free PMC article. Review.
-
Inhibition of USP7 suppresses advanced glycation end-induced cell cycle arrest and senescence of human umbilical vein endothelial cells through ubiquitination of p53.Acta Biochim Biophys Sin (Shanghai). 2022 Mar 25;54(3):311-320. doi: 10.3724/abbs.2022003. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35538032 Free PMC article.
-
Death of a Protein: The Role of E3 Ubiquitin Ligases in Circadian Rhythms of Mice and Flies.Int J Mol Sci. 2022 Sep 12;23(18):10569. doi: 10.3390/ijms231810569. Int J Mol Sci. 2022. PMID: 36142478 Free PMC article. Review.
-
CHIP induces ubiquitination and degradation of HMGB1 to regulate glycolysis in ovarian endometriosis.Cell Mol Life Sci. 2022 Dec 19;80(1):13. doi: 10.1007/s00018-022-04637-z. Cell Mol Life Sci. 2022. PMID: 36536161 Free PMC article.
References
-
- Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., and Pereira-Smith O. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 92, 9363–9367 10.1073/pnas.92.20.9363 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

