Staphylococcus aureus Fibronectin Binding Protein A Mediates Biofilm Development and Infection
- PMID: 32041788
- PMCID: PMC7171244
- DOI: 10.1128/IAI.00859-19
Staphylococcus aureus Fibronectin Binding Protein A Mediates Biofilm Development and Infection
Abstract
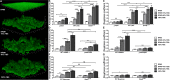
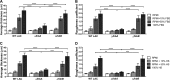
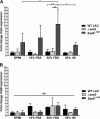
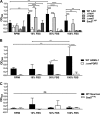
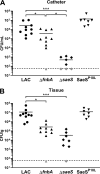
Implanted medical device-associated infections pose significant health risks, as they are often the result of bacterial biofilm formation. Staphylococcus aureus is a leading cause of biofilm-associated infections which persist due to mechanisms of device surface adhesion, biofilm accumulation, and reprogramming of host innate immune responses. We found that the S. aureus fibronectin binding protein A (FnBPA) is required for normal biofilm development in mammalian serum and that the SaeRS two-component system is required for functional FnBPA activity in serum. Furthermore, serum-developed biofilms deficient in FnBPA were more susceptible to macrophage invasion, and in a model of biofilm-associated implant infection, we found that FnBPA is crucial for the establishment of infection. Together, these findings show that S. aureus FnBPA plays an important role in physical biofilm development and represents a potential therapeutic target for the prevention and treatment of device-associated infections.
Keywords: S. aureus; biofilm; fibronectin binding protein; infection.
Copyright © 2020 American Society for Microbiology.
Figures

Similar articles
-
Staphylococcus aureus Fibronectin-Binding Protein A Mediates Cell-Cell Adhesion through Low-Affinity Homophilic Bonds.mBio. 2015 May 26;6(3):e00413-15. doi: 10.1128/mBio.00413-15. mBio. 2015. PMID: 26015495 Free PMC article.
-
SaeRS Is Responsive to Cellular Respiratory Status and Regulates Fermentative Biofilm Formation in Staphylococcus aureus.Infect Immun. 2017 Jul 19;85(8):e00157-17. doi: 10.1128/IAI.00157-17. Print 2017 Aug. Infect Immun. 2017. PMID: 28507069 Free PMC article.
-
Subdomains N2N3 of fibronectin binding protein A mediate Staphylococcus aureus biofilm formation and adherence to fibrinogen using distinct mechanisms.J Bacteriol. 2013 Jun;195(11):2675-83. doi: 10.1128/JB.02128-12. Epub 2013 Apr 5. J Bacteriol. 2013. PMID: 23564165 Free PMC article.
-
The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus.Eur J Clin Microbiol Infect Dis. 2016 Dec;35(12):1923-1931. doi: 10.1007/s10096-016-2763-0. Epub 2016 Sep 7. Eur J Clin Microbiol Infect Dis. 2016. PMID: 27604831 Review.
-
Protein-based biofilm matrices in Staphylococci.Front Cell Infect Microbiol. 2014 Dec 10;4:171. doi: 10.3389/fcimb.2014.00171. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25540773 Free PMC article. Review.
Cited by
-
Fatty acids can inhibit Staphylococcus aureus SaeS activity at the membrane independent of alterations in respiration.Mol Microbiol. 2021 Nov;116(5):1378-1391. doi: 10.1111/mmi.14830. Epub 2021 Oct 30. Mol Microbiol. 2021. PMID: 34626146 Free PMC article.
-
Antibacterial activities and action mode of anti-hyperlipidemic lomitapide against Staphylococcus aureus.BMC Microbiol. 2022 Apr 26;22(1):114. doi: 10.1186/s12866-022-02535-9. BMC Microbiol. 2022. PMID: 35473561 Free PMC article.
-
Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety.Foods. 2021 Sep 8;10(9):2117. doi: 10.3390/foods10092117. Foods. 2021. PMID: 34574227 Free PMC article. Review.
-
Prophage-encoded methyltransferase drives adaptation of community-acquired methicillin-resistant Staphylococcus aureus.bioRxiv [Preprint]. 2024 Apr 17:2024.04.17.589803. doi: 10.1101/2024.04.17.589803. bioRxiv. 2024. Update in: J Clin Invest. 2025 Jul 22:e177872. doi: 10.1172/JCI177872. PMID: 38659881 Free PMC article. Updated. Preprint.
-
First whole genome sequencing of Staphylococcus aureus isolates from Iraq: Insights into zoonotic relations and biofilm-related genes.Open Vet J. 2024 Dec;14(12):3269-3288. doi: 10.5455/OVJ.2024.v14.i12.12. Epub 2024 Dec 31. Open Vet J. 2024. PMID: 39927357 Free PMC article.
References
-
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi:10.1056/NEJMoa1306801. - DOI - PMC - PubMed

