L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling
- PMID: 32041793
- PMCID: PMC7224989
- DOI: 10.2337/db19-0764
L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling
Abstract
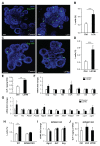
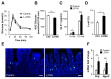
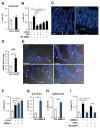
Glucagon-like peptide 1 (GLP-1) mimetics are effective drugs for treatment of type 2 diabetes, and there is consequently extensive interest in increasing endogenous GLP-1 secretion and L-cell abundance. Here we identify G-protein-coupled bile acid receptor 1 (GPBAR1) as a selective regulator of intestinal L-cell differentiation. Lithocholic acid and the synthetic GPBAR1 agonist, L3740, selectively increased L-cell density in mouse and human intestinal organoids and elevated GLP-1 secretory capacity. L3740 induced expression of Gcg and transcription factors Ngn3 and NeuroD1 L3740 also increased the L-cell number and GLP-1 levels and improved glucose tolerance in vivo. Further mechanistic examination revealed that the effect of L3740 on L cells required intact GLP-1 receptor and serotonin 5-hydroxytryptamine receptor 4 (5-HT4) signaling. Importantly, serotonin signaling through 5-HT4 mimicked the effects of L3740, acting downstream of GLP-1. Thus, GPBAR1 agonists and other powerful GLP-1 secretagogues facilitate L-cell differentiation through a paracrine GLP-1-dependent and serotonin-mediated mechanism.
© 2020 by the American Diabetes Association.
Figures

References
-
- Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell. 2017;20(2):177–190. - PubMed
-
- Petersen N, Frimurer TM, Terndrup Pedersen M, Egerod KL, Wewer Albrechtsen NJ, Holst JJ, Grapin-Botton A, Jensen KB, Schwartz TW. Inhibiting RHOA Signaling in Mice Increases Glucose Tolerance and Numbers of Enteroendocrine and Other Secretory Cells in the Intestine. Gastroenterology. 2018;155(4):1164–1176.e2. - PubMed
-
- Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2018;16(1):19–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

