Coexistence of Two Chiral Helices Produces Kink Translation in Spiroplasma Swimming
- PMID: 32041794
- PMCID: PMC7099143
- DOI: 10.1128/JB.00735-19
Coexistence of Two Chiral Helices Produces Kink Translation in Spiroplasma Swimming
Abstract
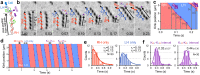
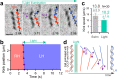
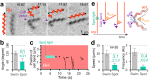
The mechanism underlying Spiroplasma swimming is an enigma. This small bacterium possesses two helical shapes with opposite-handedness at a time, and the boundary between them, called a kink, travels down, possibly accompanying the dual rotations of these physically connected helical structures, without any rotary motors such as flagella. Although the outline of dynamics and structural basis has been proposed, the underlying cause to explain the kink translation is missing. We here demonstrated that the cell morphology of Spiroplasma eriocheiris was fixed at the right-handed helix after motility was stopped by the addition of carbonyl cyanide 3-chlorophenylhydrazone (CCCP), and the preferential state was transformed to the other-handedness by the trigger of light irradiation. This process coupled with the generation and propagation of the artificial kink, presumably without any energy input through biological motors. These findings indicate that the coexistence of two chiral helices is sufficient to propagate the kink and thus to propel the cell body.IMPORTANCE Many swimming bacteria generate a propulsion force by rotating helical filaments like a propeller. However, the nonflagellated bacteria Spiroplasma spp. swim without the use of the appendages. The tiny wall-less bacteria possess two chiral helices at a time, and the boundary called a kink travels down, possibly accompanying the dual rotations of the helices. To solve this enigma, we developed an assay to determine the handedness of the body helices at the single-wind level, and demonstrated that the coexistence of body helices triggers the translation of the kink and that the cell body moves by the resultant cell bend propagation. This finding provides us a totally new aspect of bacterial motility, where the body functions as a transformable screw to propel itself forward.
Keywords: cell polarity; cytoskeleton; helical shape; motility; video microscopy.
Copyright © 2020 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

