TRAF2 Ser-11 Phosphorylation Promotes Cytosolic Translocation of the CD40 Complex To Regulate Downstream Signaling Pathways
- PMID: 32041822
- PMCID: PMC7156217
- DOI: 10.1128/MCB.00429-19
TRAF2 Ser-11 Phosphorylation Promotes Cytosolic Translocation of the CD40 Complex To Regulate Downstream Signaling Pathways
Abstract
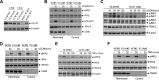
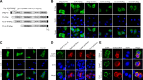
CD40 plays an important role in immune responses by activating the c-Jun N-terminal protein kinase (JNK) and NF-κB pathways; however, the precise mechanisms governing the spatiotemporal activation of these two signaling pathways are not fully understood. Here, using four different TRAF2-deficient cell lines (A20.2J, CH12.LX, HAP1, and mouse embryonic fibroblasts [MEFs]) reconstituted with wild-type or phosphorylation mutant forms of TRAF2, along with immunoprecipitation, immunoblotting, gene expression, and immunofluorescence analyses, we report that CD40 ligation elicits TANK-binding kinase 1 (TBK1)-mediated phosphorylation of TRAF2 at Ser-11. This phosphorylation interfered with the interaction between TRAF2's RING domain and membrane phospholipids and enabled translocation of the TRAF2 complex from CD40 to the cytoplasm. We also observed that this cytoplasmic translocation is required for full activation of the JNK pathway and the secondary phase of the NF-κB pathway. Moreover, we found that in the absence of Ser-11 phosphorylation, the TRAF2 RING domain interacts with phospholipids, leading to the translocation of the TRAF2 complex to lipid rafts, resulting in its degradation and activation of the noncanonical NF-κB pathway. Thus, our results provide new insights into the CD40 signaling mechanisms whereby Ser-11 phosphorylation controls RING domain-dependent subcellular localization of TRAF2 to modulate the spatiotemporal activation of the JNK and NF-κB pathways.
Keywords: CD40; JNK; NF-κB; TRAF2; phosphorylation; signaling mechanisms.
Copyright © 2020 American Society for Microbiology.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
