Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity
- PMID: 32041838
- PMCID: PMC7127976
- DOI: 10.1158/0008-5472.CAN-19-3094
Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity
Abstract
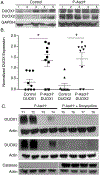
Pharmacologic ascorbate treatment (P-AscH-, high-dose, intravenous vitamin C) results in a transient short-term increase in the flux of hydrogen peroxide that is preferentially cytotoxic to cancer cells versus normal cells. This study examines whether an increase in hydrogen peroxide is sustained posttreatment and potential mechanisms involved in this process. Cellular bioenergetic profiling following treatment with P-AscH- was examined in tumorigenic and nontumorigenic cells. P-AscH- resulted in sustained increases in the rate of cellular oxygen consumption (OCR) and reactive oxygen species (ROS) in tumor cells, with no changes in nontumorigenic cells. Sources for this increase in ROS and OCR were DUOX 1 and 2, which are silenced in pancreatic ductal adenocarcinoma, but upregulated with P-AscH- treatment. An inducible catalase system, to test causality for the role of hydrogen peroxide, reversed the P-AscH--induced increases in DUOX, whereas DUOX inhibition partially rescued P-AscH--induced toxicity. In addition, DUOX was significantly downregulated in pancreatic cancer specimens compared with normal pancreas tissues. Together, these results suggest that P-AscH--induced toxicity may be enhanced by late metabolic shifts in tumor cells, resulting in a feed-forward mechanism for generation of hydrogen peroxide and induction of metabolic stress through enhanced DUOX expression and rate of oxygen consumption. SIGNIFICANCE: A high dose of vitamin C, in addition to delivering an acute exposure of H2O2 to tumor cells, activates DUOX in pancreatic cancer cells, which provide sustained production of H2O2.
©2020 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures






References
-
- Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer chemotherapy and pharmacology. 2013;71(3):765–75. - PMC - PubMed
-
- Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Science translational medicine. 2014;6(222):222ra18. - PubMed
-
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Annals of internal medicine. 2004;140(7):533–7. - PubMed
-
- Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2(−) and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer cell. 2017;32(2):268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

