Comparison of autologous osteoperiosteal cylinder and osteochondral graft transplantation in the treatment of large cystic osteochondral lesions of the talus (OLTs): a protocol for a non-inferiority randomised controlled trial
- PMID: 32041859
- PMCID: PMC7045089
- DOI: 10.1136/bmjopen-2019-033850
Comparison of autologous osteoperiosteal cylinder and osteochondral graft transplantation in the treatment of large cystic osteochondral lesions of the talus (OLTs): a protocol for a non-inferiority randomised controlled trial
Abstract
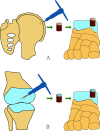
Introduction: Large cystic osteochondral lesions of the talus (OLTs) have been shown to have inferior clinical outcomes after reparative techniques such as bone marrow stimulation. Autologous osteochondral transplantation has been viewed as an alternative choice for treating these lesions, but donor-site morbidity has limited its application. Excellent clinical outcomes have been shown in repairing these types of lesions with autologous osteoperiosteal grafts, and these outcomes are achieved at a low cost and without donor-site morbidity in the normal knee joint. This will be the first randomised controlled trial to compare the two surgical techniques, and recommendations for the treatment of patients with large cystic OLTs will be provided.
Methods and analysis: A non-inferiority randomised controlled trial will be conducted. A total of 70 participants with clinically diagnosed large cystic OLTs will be randomly allocated to either the experimental group or the control group at a ratio of 1:1. The experimental group will be treated with autologous osteoperiosteal cylinder graft transplantation, while the control group will be treated with autologous osteochondral transplantation. The primary outcome measure will be the American Orthopaedic Foot & Ankle Society Ankle-Hindfoot Score and the Short Form 12 (SF-12) questionnaire. Secondary outcome measures will include the secondary arthroscopy International Cartilage Repair Society score, the Magnetic Resonance Observation of Cartilage Repair Tissue score, the Tegner activity level score, the visual analogue scale, routine X-rays, CT and complications. These parameters will be evaluated preoperatively, as well as at 3, 6, 12, 24, 36 and 60 months postoperatively. In this trial, we hypothesised that both procedures offer good results for the treatment of patients with large cystic OLTs, and occurrence of donor-site morbidity in autologous osteoperiosteal cylinder graft transplantation group is less than that in autologous osteochondral transplantation group.
Ethics and dissemination: The current study was approved by the board of research ethics of Peking University Third Hospital Medical Science Research Ethics Committee. The results of this study will be presented at national and international conferences and published in peer-reviewed journals.
Trial registration number: NCT03347877.
Keywords: Foot & ankle; osteochondral lesion; sports medicine; surgery; talus.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Comparison of Autologous Osteoperiosteal and Osteochondral Transplantation for the Treatment of Large, Medial Cystic Osteochondral Lesions of the Talus.Am J Sports Med. 2022 Mar;50(3):769-777. doi: 10.1177/03635465211068529. Epub 2022 Jan 20. Am J Sports Med. 2022. PMID: 35048728
-
Treatment of large cystic medial osteochondral lesions of the talus with autologous osteoperiosteal cylinder grafts.Arthroscopy. 2013 Aug;29(8):1372-9. doi: 10.1016/j.arthro.2013.05.014. Arthroscopy. 2013. PMID: 23906276
-
Osteoperiosteal versus osteochondral for autologous transplantation in the treatment of large cystic osteochondral lesions of the talus.J Orthop Traumatol. 2025 Feb 7;26(1):8. doi: 10.1186/s10195-025-00818-1. J Orthop Traumatol. 2025. PMID: 39918724 Free PMC article.
-
Operative treatment of osteochondral lesions of the talus.J Bone Joint Surg Am. 2013 Jun 5;95(11):1045-54. doi: 10.2106/JBJS.L.00773. J Bone Joint Surg Am. 2013. PMID: 23780543 Review.
-
Knee-to-Talus Donor-Site Morbidity Following Autologous Osteochondral Transplantation: A Meta-Analysis with Best-case and Worst-case Analysis.Clin Orthop Relat Res. 2019 Aug;477(8):1915-1931. doi: 10.1097/CORR.0000000000000719. Clin Orthop Relat Res. 2019. PMID: 31135553 Free PMC article.
Cited by
-
Knowledge, attitude and practice of patients with ankle injury regarding osteochondral lesions of the talus: a cross-sectional study in Wuxi, China.BMJ Open. 2025 Apr 3;15(4):e087402. doi: 10.1136/bmjopen-2024-087402. BMJ Open. 2025. PMID: 40180381 Free PMC article.
-
Results of arthroscopic microfracture treatment for traumatic and non-traumatic osteochondral lesions of the talus: a retrospective cohort study.BMC Musculoskelet Disord. 2025 Jul 25;26(1):709. doi: 10.1186/s12891-025-08949-6. BMC Musculoskelet Disord. 2025. PMID: 40713601 Free PMC article.
-
Effect analysis of iliac bone autografting for Hepple V osteochondral lesions of the talus.J Orthop Surg Res. 2022 Jan 15;17(1):33. doi: 10.1186/s13018-022-02924-w. J Orthop Surg Res. 2022. PMID: 35033144 Free PMC article.
-
[Osteochondral lesions of the talus : Individualized approach based on established and innovative reconstruction techniques].Unfallchirurg. 2021 Apr;124(4):319-332. doi: 10.1007/s00113-021-00964-1. Epub 2021 Mar 5. Unfallchirurg. 2021. PMID: 33666680 German.
-
A New Concept of Mosaicplasty: Autologous Osteoperiosteal Cylinder Graft Covered With Cellularized Scaffold.Arthrosc Tech. 2022 Mar 28;11(4):e655-e660. doi: 10.1016/j.eats.2021.12.033. eCollection 2022 Apr. Arthrosc Tech. 2022. PMID: 35493035 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
