Third-generation anti-CD19 chimeric antigen receptor T-cells incorporating a TLR2 domain for relapsed or refractory B-cell lymphoma: a phase I clinical trial protocol (ENABLE)
- PMID: 32041862
- PMCID: PMC7044946
- DOI: 10.1136/bmjopen-2019-034629
Third-generation anti-CD19 chimeric antigen receptor T-cells incorporating a TLR2 domain for relapsed or refractory B-cell lymphoma: a phase I clinical trial protocol (ENABLE)
Abstract
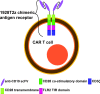
Introduction: Autologous T-cells transduced to express a chimeric antigen receptor (CAR) directed against CD19 elicit high response rates in relapsed or refractory (r/r) B-cell non-Hodgkin lymphoma (B-NHL). However, r/r B-NHL remissions are durable in fewer than half of recipients of second-generation CAR T-cells. Third-generation (3G) CARs employ two costimulatory domains, resulting in improved CAR T-cell efficacy in vitro and in animal models in vivo. This investigator-initiated, phase I dose escalation trial, termed ENABLE, will investigate the safety and preliminary efficacy of WZTL-002, comprising autologous T-cells expressing a 3G anti-CD19 CAR incorporating the intracellular signalling domains of CD28 and Toll-like receptor 2 (TLR2) for the treatment of r/r B-NHL.
Methods and analysis: Eligible participants will be adults with r/r B-NHL including diffuse large B-cell lymphoma and its variants, follicular lymphoma, transformed follicular lymphoma and mantle cell lymphoma. Participants must have satisfactory organ function, and lack other curative options. Autologous T-cells will be obtained by leukapheresis. Following WZTL-002 manufacture and product release, participants will receive lymphodepleting chemotherapy comprising intravenous fludarabine and cyclophosphamide. A single dose of WZTL-002 will be administered intravenously 2 days later. Targeted assessments for cytokine release syndrome and immune cell effector-associated neurotoxicity syndrome, graded by the American Society Transplantation and Cellular Therapy criteria, will be made. A modified 3+3 dose escalation scheme is planned starting at 5×104 CAR T-cells/kg with a maximum dose of 1×106 CAR T-cells/kg. The primary outcome of this trial is safety of WZTL-002. Secondary outcomes include feasibility of WZTL-002 manufacture and preliminary measures of efficacy.
Ethics and dissemination: Ethical approval for the study was granted by the New Zealand Health and Disability Ethics Committee (reference 19/STH/69) on 23 June 2019 for Protocol V.1.2. Trial results will be reported in a peer-reviewed journal, and results presented at scientific conferences or meetings.
Trial registration number: NCT04049513.
Keywords: B-Cell Lymphoma; CD19 Antigen; chimeric antigen receptor; clinical trial protocol; non-hodgkin lymphoma.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Trial principal investigator, RW, and co-investigator, PG, are employees of the Malaghan Institute of Medical Research, a charitable research institute and study sponsor. The other co-investigators have no competing interests to declare. PL has proprietary interest in the intellectual property of the 1928T2z construct. CB is co-Founder and Scientific Advisory Board Member of Mana Therapeutics is on the Advisory Board of Cellectis, has Stock ownership in Torque Therapeutics and Neximmune and is a Board Member of Caballeta Bio.
Figures

Similar articles
-
Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: a unicentre phase I/II clinical trial protocol.BMJ Open. 2019 May 19;9(5):e026644. doi: 10.1136/bmjopen-2018-026644. BMJ Open. 2019. PMID: 31110096 Free PMC article. Clinical Trial.
-
Efficacy and safety of third-generation CD19-CAR T cells incorporating CD28 and TLR2 intracellular domains for B-cell malignancies with central nervous system involvement: results of a pivotal trial.J Transl Med. 2025 May 27;23(1):594. doi: 10.1186/s12967-025-06608-x. J Transl Med. 2025. PMID: 40426201 Free PMC article. Clinical Trial.
-
Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol.BMJ Open. 2016 Dec 30;6(12):e013904. doi: 10.1136/bmjopen-2016-013904. BMJ Open. 2016. PMID: 28039295 Free PMC article. Clinical Trial.
-
Chimeric Antigen Receptor-Engineered T Cell Therapy in Lymphoma.Curr Oncol Rep. 2019 Mar 27;21(5):38. doi: 10.1007/s11912-019-0789-z. Curr Oncol Rep. 2019. PMID: 30919158 Review.
-
Chimeric Antigen Receptor T-Cell Therapies for Aggressive B-Cell Lymphomas: Current and Future State of the Art.Am Soc Clin Oncol Educ Book. 2019 Jan;39:446-453. doi: 10.1200/EDBK_238693. Epub 2019 May 17. Am Soc Clin Oncol Educ Book. 2019. PMID: 31099671 Review.
Cited by
-
Chimeric antigen receptor T-cell therapy for aggressive B-cell lymphomas.Front Oncol. 2024 Jul 1;14:1394057. doi: 10.3389/fonc.2024.1394057. eCollection 2024. Front Oncol. 2024. PMID: 39011476 Free PMC article. Review.
-
Immunomodulation and Immunotherapy for Patients with Prostate Cancer: An Up-to-Date Review.Biomedicines. 2025 May 12;13(5):1179. doi: 10.3390/biomedicines13051179. Biomedicines. 2025. PMID: 40427006 Free PMC article. Review.
-
Experiences and perspectives on chimeric antigen receptor (CAR) T-cell therapy among recipients, carers and referrers (RE-TELL): a qualitative study to inform CAR T-cell service design.BMJ Open. 2024 Jan 23;14(1):e071112. doi: 10.1136/bmjopen-2022-071112. BMJ Open. 2024. PMID: 38262637 Free PMC article.
-
Current and future concepts for the generation and application of genetically engineered CAR-T and TCR-T cells.Front Immunol. 2023 Mar 6;14:1121030. doi: 10.3389/fimmu.2023.1121030. eCollection 2023. Front Immunol. 2023. PMID: 36949949 Free PMC article. Review.
-
Toll-like receptor 4 signaling activation domains promote CAR T cell function against solid tumors.Mol Ther Oncol. 2024 May 14;32(2):200815. doi: 10.1016/j.omton.2024.200815. eCollection 2024 Jun 20. Mol Ther Oncol. 2024. PMID: 38840781 Free PMC article.
References
-
- Fitzmaurice C, Allen C, Barber RM, et al. . Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–48. 10.1001/jamaoncol.2016.5688 - DOI - PMC - PubMed
-
- Molina TJ, Canioni D, Copie-Bergman C, et al. . Young patients with non-germinal center B-cell-like diffuse large B-cell lymphoma benefit from intensified chemotherapy with ACVBP plus rituximab compared with CHOP plus rituximab: analysis of data from the Groupe d'Etudes des Lymphomes de l'Adulte/lymphoma study association phase III trial LNH 03-2B. J Clin Oncol 2014;32:3996–4003. 10.1200/JCO.2013.54.9493 - DOI - PubMed
-
- Récher C, Coiffier B, Haioun C, et al. . Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. The Lancet 2011;378:1858–67. 10.1016/S0140-6736(11)61040-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
