The primary mechanism of cytotoxicity of the chemotherapeutic agent CX-5461 is topoisomerase II poisoning
- PMID: 32041867
- PMCID: PMC7049172
- DOI: 10.1073/pnas.1921649117
The primary mechanism of cytotoxicity of the chemotherapeutic agent CX-5461 is topoisomerase II poisoning
Abstract
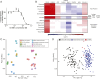
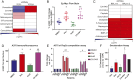
Small molecules can affect many cellular processes. The disambiguation of these effects to identify the causative mechanisms of cell death is extremely challenging. This challenge impacts both clinical development and the interpretation of chemical genetic experiments. CX-5461 was developed as a selective RNA polymerase I inhibitor, but recent evidence suggests that it may cause DNA damage and induce G-quadraplex formation. Here we use three complimentary data mining modalities alongside biochemical and cell biological assays to show that CX-5461 exerts its primary cytotoxic activity through topoisomerase II poisoning. We then show that acquired resistance to CX-5461 in previously sensitive lymphoma cells confers collateral resistance to the topoisomerase II poison doxorubicin. Doxorubicin is already a frontline chemotherapy in a variety of hematopoietic malignancies, and CX-5461 is being tested in relapse/refractory hematopoietic tumors. Our data suggest that the mechanism of cell death induced by CX-5461 is critical for rational clinical development in these patients. Moreover, CX-5461 usage as a specific chemical genetic probe of RNA polymerase I function is challenging to interpret. Our multimodal data-driven approach is a useful way to detangle the intended and unintended mechanisms of drug action across diverse essential cellular processes.
Keywords: CX-5461; chemotherapy; mechanism of action; systems biology; topoisomerase.
Conflict of interest statement
Competing interest statement: M.J.L. and J.R.P. are coinvestigators on a submitted grant.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

