Pumping the brakes on RAS - negative regulators and death effectors of RAS
- PMID: 32041893
- PMCID: PMC7033738
- DOI: 10.1242/jcs.238865
Pumping the brakes on RAS - negative regulators and death effectors of RAS
Abstract
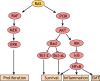
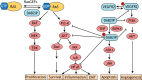
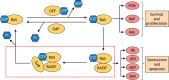
Mutations that activate the RAS oncoproteins are common in cancer. However, aberrant upregulation of RAS activity often occurs in the absence of activating mutations in the RAS genes due to defects in RAS regulators. It is now clear that loss of function of Ras GTPase-activating proteins (RasGAPs) is common in tumors, and germline mutations in certain RasGAP genes are responsible for some clinical syndromes. Although regulation of RAS is central to their activity, RasGAPs exhibit great diversity in their binding partners and therefore affect signaling by multiple mechanisms that are independent of RAS. The RASSF family of tumor suppressors are essential to RAS-induced apoptosis and senescence, and constitute a barrier to RAS-mediated transformation. Suppression of RASSF protein expression can also promote the development of excessive RAS signaling by uncoupling RAS from growth inhibitory pathways. Here, we will examine how these effectors of RAS contribute to tumor suppression, through both RAS-dependent and RAS-independent mechanisms.
Keywords: DAB2IP; GAP; NF1; RAS; RASA1; RASAL2; RASSF.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Anand S., Majeti B. K., Acevedo L. M., Murphy E. A., Mukthavaram R., Scheppke L., Huang M., Shields D. J., Lindquist J. N., Lapinski P. E. et al. (2010). MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 16, 909-914. 10.1038/nm.2186 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

