Functional Clusters of Neurons in Layer 6 of Macaque V1
- PMID: 32041896
- PMCID: PMC7083540
- DOI: 10.1523/JNEUROSCI.1394-19.2020
Functional Clusters of Neurons in Layer 6 of Macaque V1
Abstract
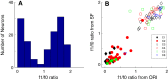
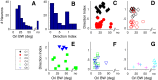
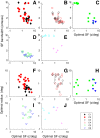
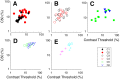
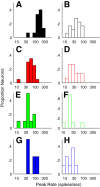
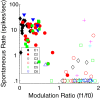
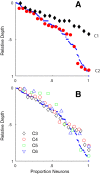
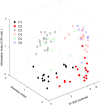
Layer 6 appears to perform a very important role in the function of macaque primary visual cortex, V1, but not enough is understood about the functional characteristics of neurons in the layer 6 population. It is unclear to what extent the population is homogeneous with respect to their visual properties or if one can identify distinct subpopulations. Here we performed a cluster analysis based on measurements of the responses of single neurons in layer 6 of primary visual cortex in male macaque monkeys (Macaca fascicularis) to achromatic grating stimuli that varied in orientation, direction of motion, spatial and temporal frequency, and contrast. The visual stimuli were presented in a stimulus window that was also varied in size. Using the responses to parametric variation in these stimulus variables, we extracted a number of tuning response measures and used them in the cluster analysis. Six main clusters emerged along with some smaller clusters. Additionally, we asked whether parameter distributions from each of the clusters were statistically different. There were clear separations of parameters between some of the clusters, particularly for f1/f0 ratio, direction selectivity, and temporal frequency bandwidth, but other dimensions also showed differences between clusters. Our data suggest that in layer 6 there are multiple parallel circuits that provide information about different aspects of the visual stimulus.SIGNIFICANCE STATEMENT The cortex is multilayered and is involved in many high-level computations. In the current study, we have asked whether there are subpopulations of neurons, clusters, in layer 6 of cortex with different functional tuning properties that provide information about different aspects of the visual image. We identified six major functional clusters within layer 6. These findings show that there is much more complexity to the circuits in cortex than previously demonstrated and open up a new avenue for experimental investigation within layers of other cortical areas and for the elaboration of models of circuit function that incorporate many parallel pathways with different functional roles.
Keywords: layer 6; primate; visual cortex.
Copyright © 2020 the authors.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
