The Sts1 nuclear import adapter uses a non-canonical bipartite nuclear localization signal and is directly degraded by the proteasome
- PMID: 32041904
- PMCID: PMC7097302
- DOI: 10.1242/jcs.236158
The Sts1 nuclear import adapter uses a non-canonical bipartite nuclear localization signal and is directly degraded by the proteasome
Abstract
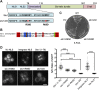
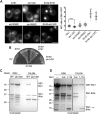
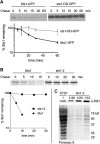
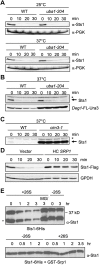
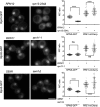
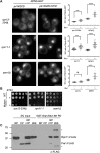
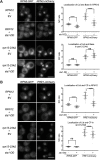
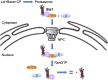
The proteasome is an essential regulator of protein homeostasis. In yeast and many mammalian cells, proteasomes strongly concentrate in the nucleus. Sts1 from the yeast Saccharomyces cerevisiae is an essential protein linked to proteasome nuclear localization. Here, we show that Sts1 contains a non-canonical bipartite nuclear localization signal (NLS) important for both nuclear localization of Sts1 itself and the proteasome. Sts1 binds the karyopherin-α import receptor (Srp1) stoichiometrically, and this requires the NLS. The NLS is essential for viability, and over-expressed Sts1 with an inactive NLS interferes with 26S proteasome import. The Sts1-Srp1 complex binds preferentially to fully assembled 26S proteasomes in vitro Sts1 is itself a rapidly degraded 26S proteasome substrate; notably, this degradation is ubiquitin independent in cells and in vitro and is inhibited by Srp1 binding. Mutants of Sts1 are stabilized, suggesting that its degradation is tightly linked to its role in localizing proteasomes to the nucleus. We propose that Sts1 normally promotes nuclear import of fully assembled proteasomes and is directly degraded by proteasomes without prior ubiquitylation following karyopherin-α release in the nucleus.
Keywords: Cut8; Karyopherin; NLS; Proteasome; Sts1; Yeast.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

