H+ Transport by K+ EXCHANGE ANTIPORTER3 Promotes Photosynthesis and Growth in Chloroplast ATP Synthase Mutants
- PMID: 32041909
- PMCID: PMC7140953
- DOI: 10.1104/pp.19.01561
H+ Transport by K+ EXCHANGE ANTIPORTER3 Promotes Photosynthesis and Growth in Chloroplast ATP Synthase Mutants
Abstract
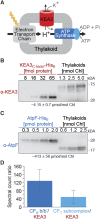
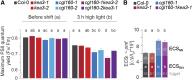
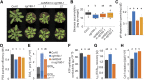
The composition of the thylakoid proton motive force (pmf) is regulated by thylakoid ion transport. Passive ion channels in the thylakoid membrane dissipate the membrane potential (Δψ) component to allow for a higher fraction of pmf stored as a proton concentration gradient (ΔpH). K+/H+ antiport across the thylakoid membrane via K+ EXCHANGE ANTIPORTER3 (KEA3) instead reduces the ΔpH fraction of the pmf. Thereby, KEA3 decreases nonphotochemical quenching (NPQ), thus allowing for higher light use efficiency, which is particularly important during transitions from high to low light. Here, we show that in the background of the Arabidopsis (Arabidopsis thaliana) chloroplast (cp)ATP synthase assembly mutant cgl160, with decreased cpATP synthase activity and increased pmf amplitude, KEA3 plays an important role for photosynthesis and plant growth under steady-state conditions. By comparing cgl160 single with cgl160 kea3 double mutants, we demonstrate that in the cgl160 background loss of KEA3 causes a strong growth penalty. This is due to a reduced photosynthetic capacity of cgl160 kea3 mutants, as these plants have a lower lumenal pH than cgl160 mutants, and thus show substantially increased pH-dependent NPQ and decreased electron transport through the cytochrome b 6 f complex. Overexpression of KEA3 in the cgl160 background reduces pH-dependent NPQ and increases photosystem II efficiency. Taken together, our data provide evidence that under conditions where cpATP synthase activity is low, a KEA3-dependent reduction of ΔpH benefits photosynthesis and growth.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures




Comment in
-
Plants Increase Photosynthesis Efficiency by Lowering the Proton Gradient across the Thylakoid Membrane.Plant Physiol. 2020 Apr;182(4):1812-1813. doi: 10.1104/pp.20.00273. Plant Physiol. 2020. PMID: 32253334 Free PMC article. No abstract available.
Similar articles
-
The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana.PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658. eCollection 2015. PLoS One. 2015. PMID: 25835989 Free PMC article.
-
Fine-tuned regulation of the K+ /H+ antiporter KEA3 is required to optimize photosynthesis during induction.Plant J. 2017 Feb;89(3):540-553. doi: 10.1111/tpj.13405. Epub 2017 Feb 3. Plant J. 2017. PMID: 27783435
-
Functional characterization of proton antiport regulation in the thylakoid membrane.Plant Physiol. 2021 Dec 4;187(4):2209-2229. doi: 10.1093/plphys/kiab135. Plant Physiol. 2021. PMID: 33742682 Free PMC article.
-
Contribution of Cyclic and Pseudo-cyclic Electron Transport to the Formation of Proton Motive Force in Chloroplasts.Mol Plant. 2017 Jan 9;10(1):20-29. doi: 10.1016/j.molp.2016.08.004. Epub 2016 Aug 26. Mol Plant. 2017. PMID: 27575692 Review.
-
Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I.Photosynth Res. 2016 Sep;129(3):253-60. doi: 10.1007/s11120-016-0227-0. Epub 2016 Feb 8. Photosynth Res. 2016. PMID: 26858094 Review.
Cited by
-
Flavodiiron proteins enhance the rate of CO2 assimilation in Arabidopsis under fluctuating light intensity.Plant Physiol. 2022 May 3;189(1):375-387. doi: 10.1093/plphys/kiac064. Plant Physiol. 2022. PMID: 35171289 Free PMC article.
-
Analyzing the effect of ion binding to the membrane-surface on regulating the light-induced transthylakoid electric potential (ΔΨm).Front Plant Sci. 2022 Jul 28;13:945675. doi: 10.3389/fpls.2022.945675. eCollection 2022. Front Plant Sci. 2022. PMID: 35968094 Free PMC article.
-
Genetically manipulated chloroplast stromal phosphate levels alter photosynthetic efficiency.Plant Physiol. 2024 Sep 2;196(1):385-396. doi: 10.1093/plphys/kiae241. Plant Physiol. 2024. PMID: 38701198 Free PMC article.
-
Proton Conduction in Zirconium-Based Metal-Organic Frameworks for Advanced Applications.ACS Appl Electron Mater. 2025 Apr 3;7(8):3164-3175. doi: 10.1021/acsaelm.5c00183. eCollection 2025 Apr 22. ACS Appl Electron Mater. 2025. PMID: 40290669 Free PMC article. Review.
-
Cytosolic and Nucleosolic Calcium-Regulated Long Non-Coding RNAs and Their Target Protein-Coding Genes in Response to Hyperosmolarity and Salt Stresses in Arabidopsis thaliana.Int J Mol Sci. 2025 Feb 27;26(5):2086. doi: 10.3390/ijms26052086. Int J Mol Sci. 2025. PMID: 40076708 Free PMC article.
References
-
- Allen J.(2002) Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 110: 273–276 - PubMed
-
- Armbruster U, Correa Galvis V, Kunz HH, Strand DD(2017) The regulation of the chloroplast proton motive force plays a key role for photosynthesis in fluctuating light. Curr Opin Plant Biol 37: 56–62 - PubMed
-
- Baker NR.(2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

