microRNA-1203 targets and silences cyclophilin D to protect human endometrial cells from oxygen and glucose deprivation-re-oxygenation
- PMID: 32041924
- PMCID: PMC7041737
- DOI: 10.18632/aging.102795
microRNA-1203 targets and silences cyclophilin D to protect human endometrial cells from oxygen and glucose deprivation-re-oxygenation
Abstract
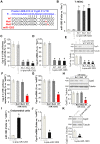
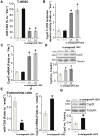
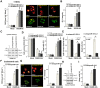
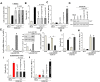
Oxygen and glucose deprivation (OGD)-re-oxygenation (OGDR) stimulation to the human endometrial cells mimics ischemia-reperfusion injury. Cyclophilin D (CypD)-dependent programmed necrosis pathway mediates OGDR-induced cytotoxicity to human endometrial cells. We here identified a novel CypD-targeting miRNA, microRNA-1203 (miR-1203). In T-HESC and primary human endometrial cells, ectopic overexpression of miR-1203, using a lentiviral construct, potently downregulated the CypD 3'-untranslated region (3'-UTR) activity and its expression. Both were however upregulated in endometrial cells with forced miR-1203 inhibition by its anti-sense sequence. Functional studies demonstrated that ectopic miR-1203 overexpression in endometrial cells alleviated OGDR-induced programmed necrosis, inhibiting mitochondrial CypD-p53-adenine nucleotide translocator 1 association, mitochondrial depolarization, reactive oxygen species production, and medium lactate dehydrogenase release. Contrarily OGDR-induced programmed necrosis and cytotoxicity were intensified with forced miR-1203 inhibition in endometrial cells. Significantly, ectopic miR-1203 overexpression or inhibition failed to change OGDR-induced cytotoxicity in CypD-knockout T-HESC cells. Furthermore, ectopic miR-1203 overexpression was unable to protect T-HESC endometrial cells from OGDR when CypD was restored by an UTR-depleted CypD construct. Collectively, these results show that miR-1203 targets and silences CypD to protect human endometrial cells from OGDR.
Keywords: cyclophilin D; human endometrial cells; miR-1203; oxygen and glucose deprivation-re-oxygenation (OGDR); programmed necrosis.
Conflict of interest statement
Figures

Similar articles
-
CPI-1189 protects neuronal cells from oxygen glucose deprivation/re-oxygenation-induced oxidative injury and cell death.Aging (Albany NY). 2021 Feb 17;13(5):6712-6723. doi: 10.18632/aging.202528. Epub 2021 Feb 17. Aging (Albany NY). 2021. PMID: 33621193 Free PMC article.
-
Ginseng Rh2 protects endometrial cells from oxygen glucose deprivation/re-oxygenation.Oncotarget. 2017 Nov 11;8(62):105703-105713. doi: 10.18632/oncotarget.22390. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285285 Free PMC article.
-
Targeting cyclophilin-D by miR-1281 protects human macrophages from Mycobacterium tuberculosis-induced programmed necrosis and apoptosis.Aging (Albany NY). 2019 Dec 28;11(24):12661-12673. doi: 10.18632/aging.102593. Epub 2019 Dec 28. Aging (Albany NY). 2019. PMID: 31884421 Free PMC article.
-
Keap1-targeting microRNA-941 protects endometrial cells from oxygen and glucose deprivation-re-oxygenation via activation of Nrf2 signaling.Cell Commun Signal. 2020 Feb 26;18(1):32. doi: 10.1186/s12964-020-0526-0. Cell Commun Signal. 2020. PMID: 32102665 Free PMC article.
-
Potential enhancement of post-stroke angiogenic response by targeting the oligomeric aggregation of p53 protein.Front Cell Neurosci. 2023 Jul 18;17:1193362. doi: 10.3389/fncel.2023.1193362. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37534043 Free PMC article. Review.
Cited by
-
Cyclophilin B serum levels present variations across the menstrual cycle.Sci Rep. 2023 Jun 22;13(1):10139. doi: 10.1038/s41598-023-37322-7. Sci Rep. 2023. PMID: 37349369 Free PMC article.
-
SphK1-targeted miR-6784 inhibits functions of skin squamous cell carcinoma cells.Aging (Albany NY). 2021 Jan 19;13(3):3726-3741. doi: 10.18632/aging.202336. Epub 2021 Jan 19. Aging (Albany NY). 2021. PMID: 33465049 Free PMC article.
-
CPI-1189 protects neuronal cells from oxygen glucose deprivation/re-oxygenation-induced oxidative injury and cell death.Aging (Albany NY). 2021 Feb 17;13(5):6712-6723. doi: 10.18632/aging.202528. Epub 2021 Feb 17. Aging (Albany NY). 2021. PMID: 33621193 Free PMC article.
-
Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction.Int J Mol Sci. 2025 May 24;26(11):5060. doi: 10.3390/ijms26115060. Int J Mol Sci. 2025. PMID: 40507871 Free PMC article. Review.
-
Implications of Activating the ANT2/mTOR/PGC-1α Feedback Loop: Insights into Mitochondria-Mediated Injury in Hypoxic Myocardial Cells.Curr Issues Mol Biol. 2023 Oct 27;45(11):8633-8651. doi: 10.3390/cimb45110543. Curr Issues Mol Biol. 2023. PMID: 37998720 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

