Immunological history governs human stem cell memory CD4 heterogeneity via the Wnt signaling pathway
- PMID: 32041953
- PMCID: PMC7010798
- DOI: 10.1038/s41467-020-14442-6
Immunological history governs human stem cell memory CD4 heterogeneity via the Wnt signaling pathway
Abstract
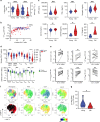
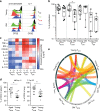
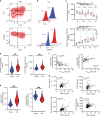
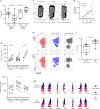
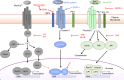
The diversity of the naïve T cell repertoire drives the replenishment potential and capacity of memory T cells to respond to immune challenges. Attrition of the immune system is associated with an increased prevalence of pathologies in aged individuals, but whether stem cell memory T lymphocytes (TSCM) contribute to such attrition is still unclear. Using single cells RNA sequencing and high-dimensional flow cytometry, we demonstrate that TSCM heterogeneity results from differential engagement of Wnt signaling. In humans, aging is associated with the coupled loss of Wnt/β-catenin signature in CD4 TSCM and systemic increase in the levels of Dickkopf-related protein 1, a natural inhibitor of the Wnt/β-catenin pathway. Functional assays support recent thymic emigrants as the precursors of CD4 TSCM. Our data thus hint that reversing TSCM defects by metabolic targeting of the Wnt/β-catenin pathway may be a viable approach to restore and preserve immune homeostasis in the context of immunological history.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

