Human cytomegalovirus promoting endothelial cell proliferation by targeting regulator of G-protein signaling 5 hypermethylation and downregulation
- PMID: 32041970
- PMCID: PMC7010708
- DOI: 10.1038/s41598-020-58680-6
Human cytomegalovirus promoting endothelial cell proliferation by targeting regulator of G-protein signaling 5 hypermethylation and downregulation
Abstract
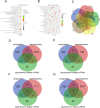
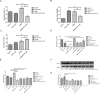
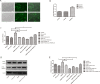
Interactions between human cytomegalovirus (HCMV) infection and environmental factors can increase susceptibility to essential hypertension (EH). Although endothelial dysfunction is the initial factor of EH, the epigenetic mechanisms through which HCMV infection induces endothelial cell dysfunction are poorly understood. Here, we evaluated whether HCMV regulated endothelial cell function and assessed the underlying mechanisms. Microarray analysis in human umbilical vein endothelial cells (HUVECs) treated with HCMV AD169 strain in the presence of hyperglycemia and hyperlipidemia revealed differential expression of genes involved in hypertension. Further analyses validated that the regulator of G-protein signaling 5 (RGS5) gene was downregulated in infected HUVECs and showed that HCMV infection promoted HUVEC proliferation, whereas hyperglycemia and hyperlipidemia inhibited HUVEC proliferation. Additionally, treatment with decitabine (DAC) and RGS5 reversed the effects of HCMV infection on HUVEC proliferation, but not triggered by hyperglycemia and hyperlipidemia. In summary, upregulation of RGS5 may be a promising treatment for preventing HCMV-induced hypertension.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Human cytomegalovirus infection-induced autophagy was associated with the biological behavioral changes of human umbilical vein endothelial cell (HUVEC).Biomed Pharmacother. 2018 Jun;102:938-946. doi: 10.1016/j.biopha.2018.03.156. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29710549
-
Human cytomegalovirus-encoded miR-UL112 contributes to HCMV-mediated vascular diseases by inducing vascular endothelial cell dysfunction.Virus Genes. 2018 Apr;54(2):172-181. doi: 10.1007/s11262-018-1532-9. Epub 2018 Jan 12. Virus Genes. 2018. PMID: 29330663
-
Increased cytomegalovirus replication by 5-Azacytidine and viral-induced cytoplasmic expression of DNMT‑1 in medulloblastoma and endothelial cells.Int J Oncol. 2018 Apr;52(4):1317-1327. doi: 10.3892/ijo.2018.4286. Epub 2018 Feb 26. Int J Oncol. 2018. PMID: 29484388
-
Human cytomegalovirus persistence and latency in endothelial cells and macrophages.Curr Opin Microbiol. 2002 Aug;5(4):403-7. doi: 10.1016/s1369-5274(02)00334-x. Curr Opin Microbiol. 2002. PMID: 12160860 Review.
-
[Interrelationship between human cytomegalovirus infection and chemokine].Nihon Rinsho. 1998 Jan;56(1):69-74. Nihon Rinsho. 1998. PMID: 9465667 Review. Japanese.
Cited by
-
Complex roles for proliferating cell nuclear antigen in restricting human cytomegalovirus replication.bioRxiv [Preprint]. 2025 Jan 6:2025.01.06.631530. doi: 10.1101/2025.01.06.631530. bioRxiv. 2025. Update in: mBio. 2025 May 14;16(5):e0045025. doi: 10.1128/mbio.00450-25. PMID: 39829879 Free PMC article. Updated. Preprint.
-
Cytomegalovirus and Cardiovascular Disease: A Hypothetical Role for Viral G-Protein-Coupled Receptors in Hypertension.Am J Hypertens. 2023 Aug 5;36(9):471-480. doi: 10.1093/ajh/hpad045. Am J Hypertens. 2023. PMID: 37148218 Free PMC article. Review.
-
Reprogramming Atherosclerosis: Precision Drug Delivery, Nanomedicine, and Immune-Targeted Therapies for Cardiovascular Risk Reduction.Pharmaceutics. 2025 Aug 7;17(8):1028. doi: 10.3390/pharmaceutics17081028. Pharmaceutics. 2025. PMID: 40871049 Free PMC article. Review.
-
miR-1929-3p Overexpression Alleviates Murine Cytomegalovirus-Induced Hypertensive Myocardial Remodeling by Suppressing Ednra/NLRP3 Inflammasome Activation.Biomed Res Int. 2020 Dec 30;2020:6653819. doi: 10.1155/2020/6653819. eCollection 2020. Biomed Res Int. 2020. PMID: 33457411 Free PMC article.
-
Identification of cellular proteins associated with human cytomegalovirus (HCMV) DNA replication suggests novel cellular and viral interactions.Virology. 2022 Jan;566:26-41. doi: 10.1016/j.virol.2021.11.004. Epub 2021 Nov 22. Virology. 2022. PMID: 34861458 Free PMC article.
References
-
- Lim SS, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
-
- Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Current topics in microbiology and immunology. 2008;325:297–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

