Down Syndrome Reduces the Sedative Effect of Midazolam in Pediatric Cardiovascular Surgical Patients
- PMID: 32041972
- PMCID: PMC7010829
- DOI: 10.1038/s41598-020-58283-1
Down Syndrome Reduces the Sedative Effect of Midazolam in Pediatric Cardiovascular Surgical Patients
Abstract
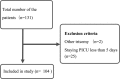
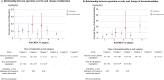
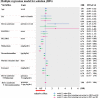
Down syndrome (DS) is frequently comorbid with congenital heart disease and has recently been shown to reduce the sedative effect of benzodiazepine (BDZ)-class anesthesia but this effect in a clinical setting has not been studied. Therefore, this study compared midazolam sedation after heart surgery in DS and normal children. We retrospectively reviewed patient records in our pediatric intensive care unit (PICU) of pediatric cardiovascular operations between March 2015 and March 2018. We selected five days of continuous post-operative data just after termination of muscle relaxants. Midazolam sedation was estimated by Bayesian inference for generalized linear mixed models. We enrolled 104 patients (average age 26 weeks) of which 16 (15%) had DS. DS patients had a high probability of receiving a higher midazolam dosage and dexmedetomidine dosage over the study period (probability = 0.99, probability = 0.97) while depth of sedation was not different in DS patients (probability = 0.35). Multi regression modeling included severity scores and demographic data showed DS decreases midazolam sedation compared with controls (posterior OR = 1.32, 95% CrI = 1.01-1.75). In conclusion, midazolam dosages should be carefully adjusted as DS significantly decreases midazolam sedative effect in pediatric heart surgery patients.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease*.Pediatr Crit Care Med. 2012 Nov;13(6):660-6. doi: 10.1097/PCC.0b013e318253c7f1. Pediatr Crit Care Med. 2012. PMID: 22791093
-
A nurse-driven analgesia and sedation protocol reduces length of PICU stay and cumulative dose of benzodiazepines after corrective surgery for tetralogy of Fallot.J Spec Pediatr Nurs. 2020 Jul;25(3):e12291. doi: 10.1111/jspn.12291. Epub 2020 Apr 3. J Spec Pediatr Nurs. 2020. PMID: 32243076
-
Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated children.Pediatr Crit Care Med. 2012 Mar;13(2):131-5. doi: 10.1097/PCC.0b013e31820aba48. Pediatr Crit Care Med. 2012. PMID: 21283046 Clinical Trial.
-
Pharmacokinetics and pharmacodynamics of midazolam given via continuous intravenous infusion in intensive care units.Clin Ther. 1997 May-Jun;19(3):405-19; discussion 367-8. doi: 10.1016/s0149-2918(97)80126-9. Clin Ther. 1997. PMID: 9220206 Review.
-
Opioid and benzodiazepine requirements in critically ill post-surgical children with down syndrome: a systematic review and meta-analysis.BMC Pediatr. 2024 Aug 7;24(1):504. doi: 10.1186/s12887-024-04971-0. BMC Pediatr. 2024. PMID: 39112949 Free PMC article.
Cited by
-
Pediatric delirium is associated with increased brain injury marker levels in cardiac surgery patients.Sci Rep. 2022 Nov 4;12(1):18681. doi: 10.1038/s41598-022-22702-2. Sci Rep. 2022. PMID: 36333387 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

