Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity
- PMID: 32041983
- PMCID: PMC7010785
- DOI: 10.1038/s41598-020-58871-1
Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity
Abstract
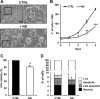
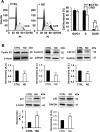
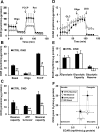
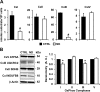
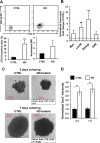
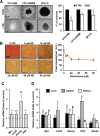
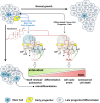
Nandrolone is a testosterone analogue with anabolic properties commonly abused worldwide, recently utilized also as therapeutic agent in chronic diseases, cancer included. Here we investigated the impact of nandrolone on the metabolic phenotype in HepG2 cell line. The results attained show that pharmacological dosage of nandrolone, slowing cell growth, repressed mitochondrial respiration, inhibited the respiratory chain complexes I and III and enhanced mitochondrial reactive oxygen species (ROS) production. Intriguingly, nandrolone caused a significant increase of stemness-markers in both 2D and 3D cultures, which resulted to be CxIII-ROS dependent. Notably, nandrolone negatively affected differentiation both in healthy hematopoietic and mesenchymal stem cells. Finally, nandrolone administration in mice confirmed the up-regulation of stemness-markers in liver, spleen and kidney. Our observations show, for the first time, that chronic administration of nandrolone, favoring maintenance of stem cells in different tissues would represent a precondition that, in addition to multiple hits, might enhance risk of carcinogenesis raising warnings about its abuse and therapeutic utilization.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Calf Spleen Extractive Injection (CSEI), a small peptides enriched extraction, induces human hepatocellular carcinoma cell apoptosis via ROS/MAPKs dependent mitochondrial pathway.J Pharmacol Sci. 2016 Oct;132(2):122-130. doi: 10.1016/j.jphs.2016.08.006. Epub 2016 Sep 4. J Pharmacol Sci. 2016. PMID: 28314430
-
Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation.Int J Mol Sci. 2021 Oct 30;22(21):11800. doi: 10.3390/ijms222111800. Int J Mol Sci. 2021. PMID: 34769230 Free PMC article.
-
Millepachine, a novel chalcone, induces G2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo.Carcinogenesis. 2013 Jul;34(7):1636-43. doi: 10.1093/carcin/bgt087. Epub 2013 Mar 7. Carcinogenesis. 2013. PMID: 23471882
-
Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature.Br J Sports Med. 2005 May;39(5):e27. doi: 10.1136/bjsm.2004.013599. Br J Sports Med. 2005. PMID: 15849280 Free PMC article. Review.
-
The impact of nandrolone decanoate on the central nervous system.Curr Neuropharmacol. 2015 Jan;13(1):122-31. doi: 10.2174/1570159X13666141210225822. Curr Neuropharmacol. 2015. PMID: 26074747 Free PMC article. Review.
Cited by
-
The Impact of Nandrolone Decanoate in the Osseointegration of Dental Implants in a Rabbit Model: Histological and Micro-Radiographic Results.Materials (Basel). 2021 Apr 27;14(9):2258. doi: 10.3390/ma14092258. Materials (Basel). 2021. PMID: 33925604 Free PMC article.
-
Anabolic androgenic steroid-induced liver injury: An update.World J Gastroenterol. 2022 Jul 14;28(26):3071-3080. doi: 10.3748/wjg.v28.i26.3071. World J Gastroenterol. 2022. PMID: 36051334 Free PMC article. Review.
-
The Molecular Quality and Mitochondrial Activity of Porcine Cumulus-Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions.Int J Mol Sci. 2022 Apr 20;23(9):4572. doi: 10.3390/ijms23094572. Int J Mol Sci. 2022. PMID: 35562963 Free PMC article.
-
Forensic Post-Mortem Investigation in AAS Abusers: Investigative Diagnostic Protocol. A Systematic Review.Diagnostics (Basel). 2021 Jul 21;11(8):1307. doi: 10.3390/diagnostics11081307. Diagnostics (Basel). 2021. PMID: 34441242 Free PMC article. Review.
-
miRNAs as Novel Biomarkers of Chronic Kidney Injury in Anabolic-Androgenic Steroid Users: An Experimental Study.Front Pharmacol. 2020 Sep 16;11:563756. doi: 10.3389/fphar.2020.563756. eCollection 2020. Front Pharmacol. 2020. PMID: 33041804 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

